The known Snake antivenom has been available in Australia for decades, but information on clinical outcomes, antivenom treatment and deaths caused by snake envenoming is still limited.
The new The recommendation to administer one vial of antivenom for bites by major snake groups has been widely adopted, with no evidence of adverse consequences for patients. The death rate for snakebite in Australia and the average interval between bite and first administration of antivenom did not change during 2005–2015.
The implications Research and clinical practice should focus on improving early diagnosis, enabling earlier administration of antivenom.
Venomous snakes are found in every Australian state and territory and in the warmer waters surrounding the continent.1 Most medically important Australian snakes belong to five genera of terrestrial snakes: brown snakes (Pseudonaja spp.), tiger snakes (Notechis spp.), taipans (Oxyuranus spp.), death adders (Acanthophis spp.) and black snakes (Pseudechis spp.). It was previously estimated that up to 3000 people were bitten by snakes in Australia each year. However, this number is not based on any reported epidemiological study, and would include unverified estimates of suspected bites, dry bites and “stick” bites.1 Although the number of cases requiring antivenom is unclear,1 one to four deaths are attributed to snakebite annually.2,3 Bites by sea snakes are extremely rare in Australia.
Antivenom has been available in Australia from the Commonwealth Serum Laboratories since 1930 (since 2015: from Seqirus).4 No controlled clinical trials of Australian snake antivenoms have been published. Product information and guidelines supplied by the manufacturer5 contain treatment advice based on single case reports and expert opinion. Older published case series of Australian snakebite6-13 were generally limited to small numbers in particular regions, were retrospective, included cases for which the snake was not definitively identified, and had no method for quantifying the effects of antivenom.
Clinical practice gradually shifted during the 1980s and 1990s to escalating doses of antivenom, but without clinical evidence of a change in outcomes, particularly for brown snake envenoming.14 Antivenom is expensive and can elicit systemic hypersensitivity reactions.4,15 Growing awareness of unanswered questions about the clinical effects of Australian snakebites and the effectiveness and safety of antivenom led to the need for a prospective, Australia-wide study of snakebite being recognised.16 In this article we report our findings on 10 years of snakebites in Australia. We describe the clinical effects of snakebites and their treatment, and highlight recent changes in practice.
Methods
The Australian Snakebite Project (ASP) is a prospective, multicentre observational study that recruits patients with suspected snakebite. All patients presenting to an Australian hospital with suspected or confirmed snakebites from July 2005 to June 2015 were eligible for recruitment by ASP investigators after being identified by telephone calls to the National Poisons Information Centre Network or to a national free call study number, by local investigators at many hospitals, or by state laboratory services. For envenomed patients, the snake was identified by venom-specific enzyme immunoassays for all major Australian snakes, or by a licensed reptile handler or zoo or museum snake expert. Venom-specific enzyme immunoassays of blood samples used biotinylated antibodies and streptavidin–horseradish peroxidase as detecting agents (limit of detection: 0.1–0.2 ng/mL).17
Demographic data and information on the circumstances of the bite, its clinical effects, investigations (including Seqirus snake venom detection kit [SVDK] test results), complications and treatment were collected for each recruited patient. Datasheets and patient information and consent forms were faxed to the hospital, filled by treating clinicians, and returned by fax to the investigators. Hospital medical records were obtained to fill in missing data. A trained research assistant entered the data into a relational database (Microsoft Access) that was reviewed by the chief investigator (GKI). Treatment was determined by the hospital, often in consultation with the local Poisons Information Centre or a clinical toxicologist. Clinical syndromes of snake envenoming were defined as previously reported, based on serial clinical features and laboratory investigations (online Appendix, table 1).18 Patients were deemed to be non-envenomed if they did not manifest any clinical envenoming syndrome while in hospital (until at least 12 hours after the bite) and did not return to hospital with features of delayed envenoming.19
The SVDK is a commercial bedside test for bite site samples, or for urine samples if a bite site sample is not available. The test is less reliable for urine samples, and is not recommended for testing blood. The SVDK result was deemed inconclusive if results for both bite site and urine samples were available but contradictory. Systemic hypersensitivity reactions to antivenom administration were defined as either skin-only reactions (urticaria, angioedema) if there was no evidence of other organ involvement, or as anaphylaxis if there were multisystem effects according to the National Institute of Allergy and Infectious Disease – Food Allergy and Anaphylaxis Network (NIAID-FAAN) consensus criteria for anaphylaxis.20 We defined reaction severity according to the Brown grading system.21
The National Coronial Information System (NCIS) database, widely employed in public health research,22 was retrospectively searched on 10 November 2015 for closed cases during the study period that included “snakebite” in the cause of death field. A keyword search of attached documentation was not performed. Cases were manually reviewed for inclusion.
Statistical analysis
In this article we report the demographic features of patients, the circumstances of the bites, the clinical effects of envenoming, and the results of laboratory investigations and SVDK testing; we also report on antivenom treatment and adverse reactions, time to discharge, and deaths.
Continuous outcomes are reported as medians (with interquartile ranges [IQRs]) and ranges. Continuous outcomes, including time to antivenom administration, antivenom dose, and time to hospital discharge in different years (ie, ten groups) were compared in Kruskal–Wallis tests. All analyses were conducted in GraphPad Prism 7.01 for Windows (GraphPad Software).
Ethics approval
Human research ethics committee (HREC) approval for the study was obtained from major state and territory HRECs, including those of the Northern Territory Department of Health and Menzies School of Health Research (reference, 04/08), the Hunter New England Area Health Service and the University of Newcastle (reference, 07/11/21/3.06), the Royal Perth Hospital Ethics Committee and South Metro Area Health Service (reference, RA-08/003), the Western Australian Country Health Service (reference, 2008:03), the Tasmania Network (reference, H00109965), and the Gold Coast Health Service District (reference, 200835), as well as for a further ten HRECs of participating facilities. Informed consent was obtained from all enrolled patients, or from a parent or guardian for participants under 18 years of age.
Results
During July 2005 – June 2015, 1548 patients were recruited from 171 hospitals in all Australian states and territories (online Appendix, figures 1 and 2); 282 (18%) were recruited from remote/rural hospitals, 605 (39%) from regional hospitals, 211 (14%) from metropolitan hospitals, and 450 (29%) from tertiary hospitals. Recruitment was relatively constant (median, 152 cases/year; IQR, 143–167), with a seasonal increase during warmer months (Box 1). The median patient age was 38 years (IQR, 23–53 years; range, 1–92 years); 1135 (73%) were male, 168 (11%) were snake handlers, and 54 (3.5%) were affected by alcohol at the time of the bite. The bite site was the lower limb in 813 cases (52%), upper limb in 646 (42%), torso in 13 (0.8%), head in two (0.1%), and unknown in 74 cases (4.8%). The bite occurred while walking or during other activity unknowingly near a snake in 730 cases (47.1%), while attempting to catch or kill the snake in 224 (14%), and while gardening in 128 (8.3%) (online Appendix, table 2). Snakebites occurred near houses (485 cases, 31%), inside buildings (220 cases, 14%), or in bush or scrubland (172 cases, 11%) (online Appendix, table 3).
Pressure bandages with immobilisation (PBI) were applied to 1304 patients (84%). The rate of complications varied between those receiving and not receiving PBI, but not markedly nor consistently in association with particular snake types or complications.
Systemic envenoming occurred in 835 patients (54%; median, 87 patients per year; IQR, 62–102 per year; Box 1). Demographic and bite details for envenomed and non-envenomed patients were similar, except that snake handlers and intoxicated patients who were bitten were much more likely to be envenomed than not (online Appendix, table 4). The snake type was accurately determined in 718 envenomed cases (86%): 448 by venom-specific enzyme immunoassay of blood, 270 by expert identification. Of these, the most common snakes involved were brown snakes (41% of envenomed cases), tiger snakes (17%), and red-bellied black snakes (Pseudechis porphyriacus; 16%) (online Appendix, figure 3).
Clinical effects
The most common systemic envenoming syndrome in the 835 envenomed patients was venom-induced consumption coagulopathy (611 cases, 73%), myotoxicity (142 cases, 17%), acute kidney injury (97 cases, 12%), and neurotoxicity (83 cases, 10%). Microangiopathic haemolytic anaemia occurred in 66 cases (7.9%). Less common but more severe complications included cardiac arrest in 25 cases (2.9%) and major haemorrhage in 13 (1.6%), both mainly after brown snake envenoming. The proportion of systemic envenoming syndromes in the 718 patients with accurate snake identification is shown in Box 2.
Deaths
There were 23 deaths, a median of two per year (range, 0–6; Box 3, online Appendix, figure 4); three were snake handlers. Out-of-hospital cardiac arrest was the most common cause of death (ten cases), followed by intracranial haemorrhage (six cases). Seventeen deaths were attributed to brown snakes, four to tiger snakes, and two to unknown snake types. In seven of 11 deaths by cardiac arrest, PBI had not been applied before the patient collapsed; the median time to cardiopulmonary resuscitation (CPR) was 15 minutes (range, 1–45 min). PBI was applied to 10 of 12 cardiac arrest patients who survived, with a median time to CPR of one minute (range, 1–10 min).
Snake venom detection kits
SVDKs were used in 1060 cases (68%), including 695 envenomed patients (83% of envenomed patients); bite site samples were tested in 486 cases, urine in 79 cases, and both in 130 cases. For 597 envenomed cases in which the snake type was confirmed and an SVDK test performed, the result was positive for an incorrect snake in 29 cases (4.9%), negative in 50 (8.4%), and inconclusive in 22 (3.7%) (Box 4). An SVDK test was performed for 364 non-envenomed patients (51%); bite site samples were tested in 259 cases, urine in 37 cases, and both in 68 cases. The result was positive for 133 patients (36% of those tested), of whom nine received antivenom because of this result (online Appendix, tables 5 and 6).
Antivenom treatment
Antivenom was administered to 755 patients, including 49 who had not been envenomed (online Appendix, table 6). The first dose of antivenom was tiger snake antivenom in 319 cases (42%), brown snake in 279 (37%), polyvalent in 81 (11%), black snake in 32 (4%), taipan in 23 (3%), death adder in 17 (2%), and sea snake antivenom in four (0.5%). The median time between bite and first administration of antivenom was 4.3 hours (IQR, 2.7–6.3 h; range, 0.25–80 h); this interval did not change significantly over the ten years (range, 3.7–4.5 h; P = 0.57; online Appendix, figure 5). However, the median total antivenom dose dropped significantly between 2005 and 2015, from a median of four vials to one (P < 0.001), mainly because of reduced doses of brown snake, tiger snake and taipan antivenom (Box 5).
Immediate systemic hypersensitivity reactions were experienced by 178 patients who received antivenom (24%), including skin-only systemic hypersensitivity reactions in 94 cases (13%), anaphylaxis in 39 (5%), and severe anaphylaxis in 45 (6%). Of the patients with severe anaphylaxis, 32 had hypotension, four hypoxia, seven hypotension and hypoxia, and two altered consciousness, but there were no deaths. Adrenaline was administered to 76 patients, antihistamines to 76, and corticosteroids to 27. Ten non-envenomed patients had adverse reactions to antivenom (6% of all patients who experienced hypersensitivity reactions), four of which were severe. The proportions of patients with hypersensitivity reactions varied from year to year, but with no consistent trend (online Appendix, figure 6).
Time to hospital discharge
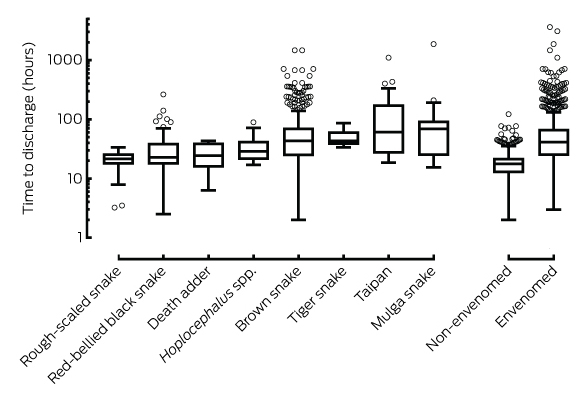
Time from bite to discharge from hospital was longer for envenomed patients (median, 41 h; IQR, 25–67 h; range, 3–3613 h) than for non-envenomed patients (median, 18 h; IQR, 13–22 h; range, 2–122 h; Box 6). The interval was shortest for patients with bites by the rough-scaled snake (Tropidechis carinatus) (median, 22 h; IQR, 18–26 h) and longest for those with mulga snake (Pseudechis australis) envenoming (median, 69 h; IQR, 26–93 h; Box 6). The median time to discharge for envenomed patients did not differ significantly over the ten years, ranging between 37 and 42 hours (P = 0.74; online Appendix, figure 7).
Discussion
We have described the clinical spectrum of snake envenoming and changes in treatment for snakebite in Australia during 2005–15. Most clinical effects were consistent with earlier studies,18,23-27 except that acute kidney injury and microangiopathic haemolytic anaemia were more common than reported by previous studies.1,6-10 The fatality rate (two deaths per year) was similar to that for previous decades.2,3 The SVDK was inappropriately used in non-envenomed patients, and yielded inconclusive, negative or incorrect results for 17% of envenomed patients (101 of 597). Over the ten years, the amount of antivenom administered declined markedly, but the interval between bite and first antivenom has remained constant. PBI did not consistently improve outcomes, but other factors that influence outcomes need to be explored, as do time to presentation and antivenom administration, and the quality and timing of PBI.
Two snakebite deaths annually is low for our population size, but the fatality rate among envenomed patients (23 of 835, 2.8%) is relatively high. Almost half the deaths were caused by out-of-hospital cardiac arrests, which may explain why the annual death rate has not changed. The median time to CPR was much shorter for patients surviving cardiac arrest after snakebite, although they had more frequently also had PBI before they collapsed. Improved pre-hospital care, including early, effective basic life support, may be more important than changes in hospital care or antivenom treatment, and could be assisted by public education.
Our results cast further doubt on the usefulness of SVDK testing. A recent study of the SVDK, using non-patient samples analysed under laboratory conditions, found a low false positive rate.28 We examined its real world application and interpretation in patient care; a false positive SVDK result motivated antivenom administration to nine of 49 non-envenomed patients who had the test performed (18%), placing them at unnecessary risk of adverse reactions.
The median antivenom dose administered to patients decreased over the ten years, particularly for bites by snakes that cause venom-induced consumption coagulopathy, probably reflecting changes to the recommended treatment of envenoming by Australian snakes.29 Antivenom has a shelf-life of only 1–3 years, and costs $347–2320 per vial; as an emergency treatment, it needs to be widely available, and about 750 Australian hospitals stock antivenom. Confirmation that one vial is sufficient should lead both to an increase in very small hospitals stocking antivenom and tens of millions of dollars being saved each year because larger hospitals can stock less.
The median time to antivenom administration was relatively constant, despite increasing evidence for the value of early administration.18,25,26 Reducing this interval is an important and achievable goal for improving treatment, but there is a risk of eliciting anaphylaxis in non-envenomed patients. Reducing the time to antivenom administration therefore also requires improving the early diagnosis and recognition of systemic envenoming, which is often undertaken in small or remote health care facilities with minimal specialist support. Further investigations should focus on clinical symptoms, and perhaps novel biomarkers and alternative bedside investigations, which can help guide clinicians with respect to early antivenom administration.30
Limitations to our study
It is difficult to explore treatment effects in observational studies, although it is clear that neither death nor complication rates have increased with lower antivenom doses. Voluntary participation by health care staff meant that only cases notified to the investigators were recruited. Some patients may have been missed because they were not identified, particularly non-envenomed patients and people with unconfirmed snakebites, as well as a small proportion (under 2%) who did not consent to participate. There is also a recruitment bias favouring patients with more severe or unusual envenoming syndromes, as reflected by the high envenoming rate (over 50%). However, the number of cases recruited accounts for a very large proportion of the estimated total number of snakebites in Australia. Fatal cases may have been missed if patients died outside hospital and were not identified as snakebite deaths by the coroner.
Conclusion
Snake envenoming is infrequent in Australia, and much less common than previous annual estimates suggested. It is often severe and the annual death rate did not change during the ten years assessed by the study. PBI continues to be applied; despite a lack of strong evidence that it improves outcomes, it remains a safe first aid approach. The SVDK was used inappropriately in non-envenomed patients (with a 36% false positive rate), and its results were also incorrect in 17% of envenomed patients; it is consequently no longer recommended as an essential component of snakebite assessment. As the usual antivenom dose for all major snake groups has decreased to one vial, with no evidence of adverse consequences, this approach should be retained; stocking antivenom is expensive, and lower doses will save millions of dollars each year. Long delays in administering antivenom remain common; better diagnostic and management strategies that rapidly identify envenomed patients are required in order to expedite antivenom administration.
Expert advice on snakebite treatment is available from the National Poisons Information Centre Network (telephone: 13 11 26 from anywhere in Australia).
Box 1 – Recruitment of patients with snakebite, Australia, July 2005 – June 2015, by month*
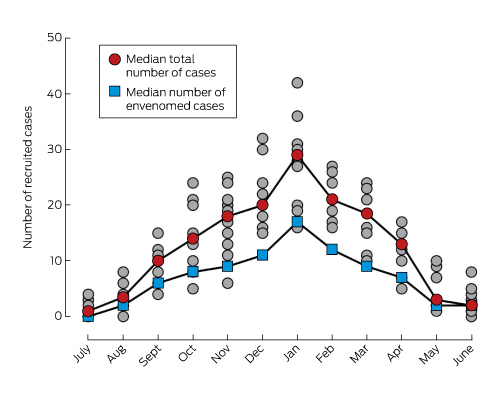
* Grey circles: total numbers of cases recruited in individual years.
Box 2 – Envenoming syndromes for each snake type in the 718 cases where the type was definitively identified
|
Snake type |
Envenomed cases |
VICC |
AC |
Neuro-toxicity |
Myo-toxicity |
Acute kidney injury |
MAHA |
Cardiac arrest |
Major haemorrhage |
NSSS |
Deaths* |
||||
|
|
|||||||||||||||
|
Brown snake |
296 (41%) |
292 (99%) |
0 |
0 |
13 (4%) |
53 (18%) |
41 (14%) |
19 (6%) |
10 (3%) |
119 (40%) |
15 (5%) |
||||
|
Tiger snake |
119 (16%) |
113 (95%) |
0 |
31 (26%) |
42 (35%) |
13 (11%) |
7 (6%) |
3 (3%) |
2 (2%) |
81 (68%) |
4 (3%) |
||||
|
Red-bellied black snake |
117 (16%) |
0 |
54 (46%) |
0 |
23 (20%) |
0 |
0 |
0 |
0 |
107 (91%) |
0 |
||||
|
Rough-scaled snake |
64 (9%) |
62 (97%) |
0 |
4 (6%) |
12 (19%) |
5 (8%) |
0 |
0 |
1 (2%) |
48 (75%) |
0 |
||||
|
Taipan |
31 (4%) |
28 (90%) |
0 |
17 (55%) |
11 (35%) |
12 (39%) |
8 (26%) |
2 (6%) |
0 |
26 (84%) |
0 |
||||
|
Mulga snake |
31 (4%) |
0 |
21 (68%) |
0 |
17 (55%) |
1 (3%) |
0 |
0 |
0 |
28 (90%) |
0 |
||||
|
Hoplocephalus spp. |
27 (4%) |
27 (100%) |
0 |
0 |
2 (7%) |
2 (7%) |
2 (7%) |
0 |
0 |
16 (59%) |
0 |
||||
|
Death adder |
21 (3%) |
0 |
0 |
15 (71%) |
2 (10%) |
0 |
0 |
0 |
0 |
12 (57%) |
0 |
||||
|
Sea snake |
6 (1%) |
0 |
0 |
0 |
2 (33%) |
0 |
0 |
0 |
0 |
4 (67%) |
0 |
||||
|
Collett’s snake |
3 (0.4%) |
0 |
3 (100%) |
0 |
1 (33%) |
0 |
0 |
0 |
0 |
3 (100%) |
0 |
||||
|
Total |
715 |
522 (73%) |
68 (10%) |
67 (9%) |
125 (17%) |
86 (12%) |
58 (8%) |
24 (3%) |
13 (2%) |
444 (62%) |
19 (3%) |
||||
|
|
|||||||||||||||
|
AC = anticoagulant coagulopathy; MAHA = microangiopathic haemolytic anaemia; NSSS = non-specific systemic symptoms; VICC = venom-induced consumption coagulopathy (complete or partial). * Includes only deaths of patients recruited to the Australian Snakebite Project (ASP) (ie, excludes cases in National Coronial Information System not concerning patients recruited to the ASP). Two confirmed bites by whip snakes (Demansia spp.) and one by an ornamental snake (Denisonia maculata) with envenoming are not included in the table. |
|||||||||||||||
Box 3 – Details for 23 deaths attributed to snakebite, recorded by the Australian Snakebite Project (ASP) or the National Coronial Information System (NCIS)
|
Age/sex |
Season |
Location |
Snake type |
Cause of death |
Ante mortem venom concentration |
NCIS |
ASP |
Time to antivenom (hours) |
Total antivenom dose (vials) |
||||||
|
|
|||||||||||||||
|
43/F |
2005/06 |
Rural |
Brown snake |
Out-of-hospital cardiac arrest |
0.4 ng/mL |
|
Yes |
3.5 |
11 |
||||||
|
28/M |
2005/06 |
Rural |
Unknown |
Unknown |
Unknown |
Yes |
|
Unknown |
Unknown |
||||||
|
20/M |
2005/06 |
Rural |
Brown snake |
Hyperthermia, multi-organ failure* |
1.2 ng/mL |
Yes |
Yes |
15.0 |
6 |
||||||
|
45/M |
2006/07 |
Regional |
Brown snake |
Out-of-hospital cardiac arrest |
0.1 ng/mL |
Yes |
Yes |
3.3 |
1 |
||||||
|
16/M |
2006/07 |
Rural |
Brown snake |
Out-of-hospital cardiac arrest |
156 ng/mL |
Yes |
Yes |
1.2 |
2 |
||||||
|
46/M |
2006/07 |
Rural |
Brown snake |
Out-of-hospital cardiac arrest |
Unknown |
Yes |
|
Unknown |
Unknown |
||||||
|
10/F |
2006/07 |
Rural |
Brown snake |
Out-of-hospital cardiac arrest |
3 ng/mL |
|
Yes |
1.7 |
6 |
||||||
|
37/M |
2006/07 |
Rural |
Unknown |
Unknown |
Unknown |
Yes |
|
Unknown |
Unknown |
||||||
|
69/F |
2008/09 |
Rural |
Brown snake |
Intracranial haemorrhage |
3.5 ng/mL |
|
Yes |
1.3 |
2 |
||||||
|
38/M |
2008/09 |
Urban |
Brown snake |
Unknown |
Unknown |
Yes |
|
Unknown |
Unknown |
||||||
|
72/M |
2009/10 |
Rural |
Tiger snake |
Acute kidney injury and overwhelming rhabdomyolysis |
Unknown |
Yes |
Yes |
7.5 |
2 |
||||||
|
61/F |
2010/11 |
Rural |
Brown snake |
Out-of-hospital cardiac arrest |
1.5 ng/mL |
Yes |
Yes |
1.4 |
5 |
||||||
|
43/M |
2010/11 |
Rural |
Brown snake |
Out-of-hospital cardiac arrest |
Unknown |
|
Yes |
1.5 |
4 |
||||||
|
43/M |
2011/12 |
Rural |
Brown snake |
Out-of-hospital cardiac arrest |
Unknown |
|
Yes |
1.1 |
1 |
||||||
|
35/M |
2011/12 |
Regional |
Brown snake |
Out-of-hospital cardiac arrest |
Unknown |
Yes |
Yes |
0.4 |
4 |
||||||
|
26/M |
2012/13 |
Rural |
Brown snake |
Out-of-hospital cardiac arrest |
Unknown |
|
Yes |
2.2 |
1 |
||||||
|
80/M |
2012/13 |
Rural |
Brown snake |
Intracranial haemorrhage |
4.6 ng/mL |
|
Yes |
3.2 |
1 |
||||||
|
72/M |
2012/13 |
Rural |
Brown snake |
Intracranial haemorrhage |
11.5 ng/mL |
|
Yes |
3.5 |
1 |
||||||
|
61/M |
2012/13 |
Rural |
Tiger snake |
Intracranial haemorrhage |
0.3 ng/mL |
|
Yes |
8.1 |
1 |
||||||
|
26/M |
2012/13 |
Urban |
Brown snake |
Intracranial haemorrhage |
7.6 ng/mL |
|
Yes |
2.8 |
2 |
||||||
|
64/M |
2012/13 |
Rural |
Tiger snake |
Major trauma while coagulopathic† |
20 ng/mL |
|
Yes |
0 |
0 |
||||||
|
57/F |
2013/14 |
Rural |
Brown snake |
Intracranial haemorrhage |
9.6 ng/mL |
|
Yes |
9.6 |
1 |
||||||
|
27/M |
2014/15 |
Rural |
Tiger snake |
Overwhelming rhabdomyolysis |
26 ng/mL |
|
Yes |
0.5 |
2 |
||||||
|
|
|||||||||||||||
|
* The patient collapsed after a snakebite following an afternoon run while the outside temperature was 46°C. † The patient was involved in a motor vehicle accident after being bitten by a snake. |
|||||||||||||||
Box 4 – Snake venom detection kit (SVDK; Seqirus) results for samples from envenomed patients in 597 cases for which the snake type was confirmed*
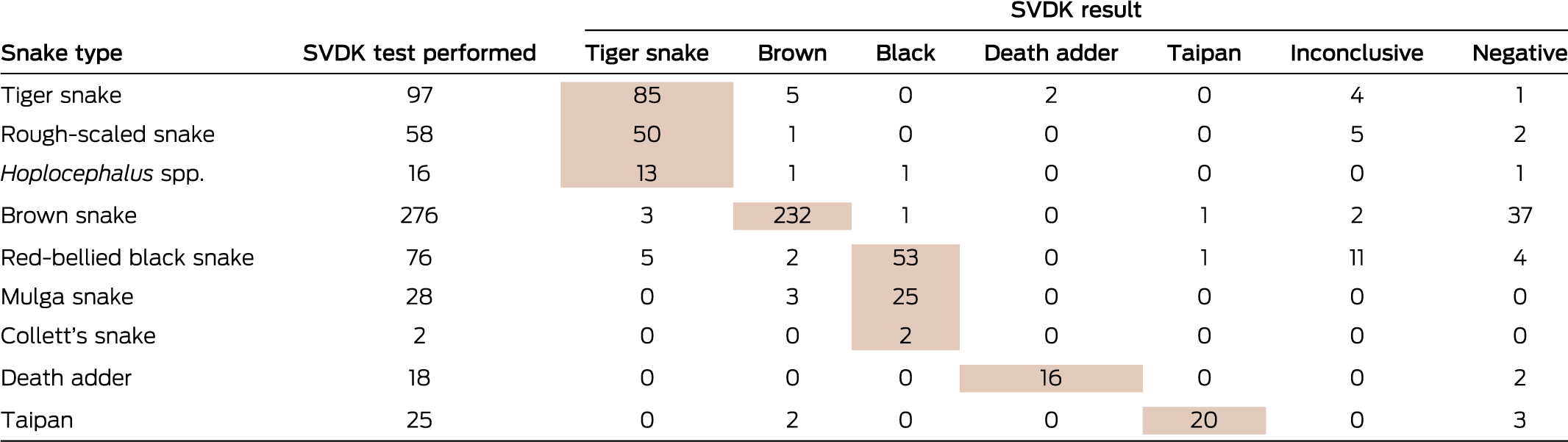
* Shading: SVDK test result consistent with the confirmed snake identification. For one bite by a confirmed ornamental snake, there was a positive SVDK result in the black snake well (incorrect snake).
Box 5 – Median total dose of antivenom (with upper quartile) for six major snake groups, by season*
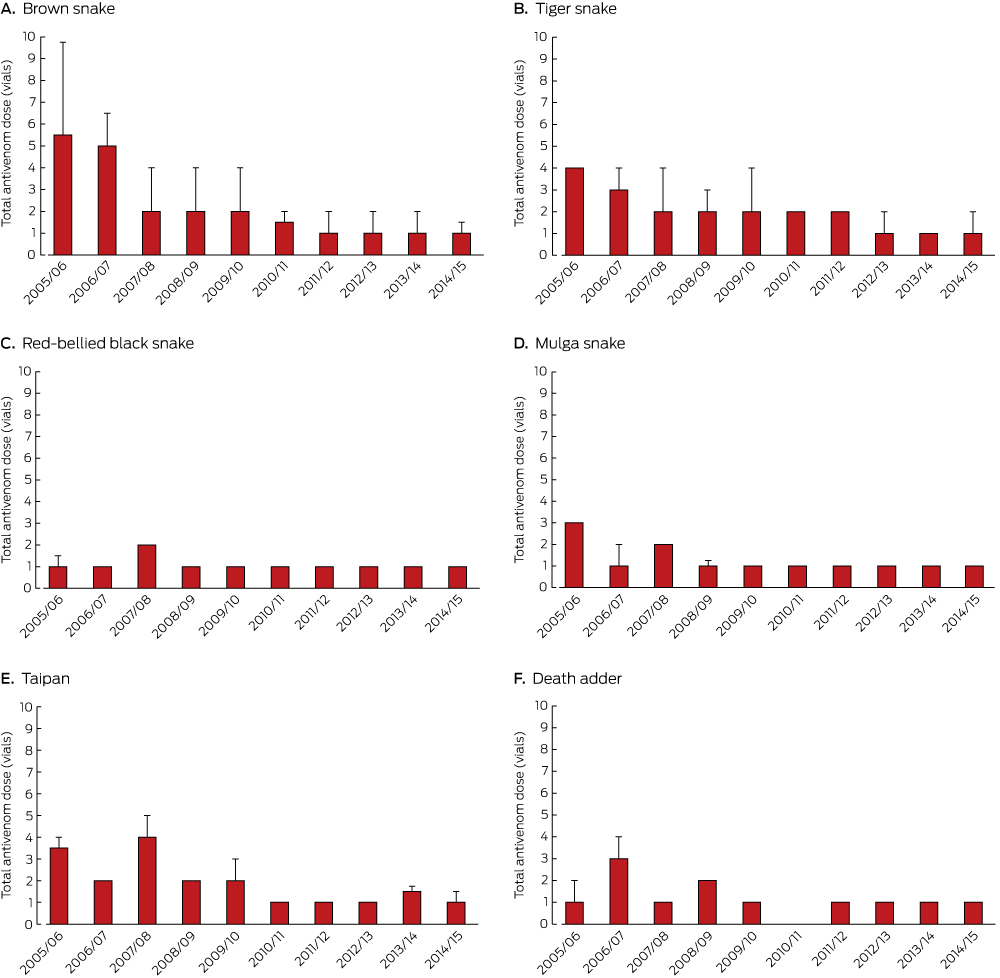
* One vial of polyvalent antivenom was deemed equivalent to one vial of monovalent antivenom.5
Received 31 January 2017, accepted 8 June 2017





Abstract
Objective: To describe the epidemiology, treatment and adverse events after snakebite in Australia.
Design: Prospective, multicentre study of data on patients with snakebites recruited to the Australian Snakebite Project (2005–2015) and data from the National Coronial Information System.
Setting, participants: Patients presenting to Australian hospitals with suspected or confirmed snakebites from July 2005 to June 2015 and consenting to participation.
Main outcome measures: Demographic data, circumstances of bites, clinical effects of envenoming, results of laboratory investigations and snake venom detection kit (SVDK) testing, antivenom treatment and adverse reactions, time to discharge, deaths.
Results: 1548 patients with suspected snakebites were enrolled, including 835 envenomed patients (median, 87 per year), for 718 of which the snake type was definitively established, most frequently brown snakes (41%), tiger snakes (17%) and red-bellied black snakes (16%). Clinical effects included venom-induced consumption coagulopathy (73%), myotoxicity (17%), and acute kidney injury (12%); severe complications included cardiac arrest (25 cases; 2.9%) and major haemorrhage (13 cases; 1.6%). There were 23 deaths (median, two per year), attributed to brown (17), tiger (four) and unknown (two) snakes; ten followed out-of-hospital cardiac arrests and six followed intracranial haemorrhages. Of 597 SVDK test results for envenomed patients with confirmed snake type, 29 (4.9%) were incorrect; 133 of 364 SVDK test results for non-envenomed patients (36%) were false positives. 755 patients received antivenom, including 49 non-envenomed patients; 178 (24%), including ten non-envenomed patients, had systemic hypersensitivity reactions, of which 45 (6%) were severe (hypotension, hypoxaemia). Median total antivenom dose declined from four vials to one, but median time to first antivenom was unchanged (4.3 hours; IQR, 2.7–6.3 hours).
Conclusions: Snake envenoming is uncommon in Australia, but is often severe. SVDKs were unreliable for determining snake type. The median antivenom dose has declined without harming patients. Improved early diagnostic strategies are needed to reduce the frequently long delays before antivenom administration.