There is conflicting advice about the appropriate dosing of antivenom for patients envenomed by brown snakes in Australia, with recommended initial doses ranging from one to four ampoules.1-3
Determining the dose required to neutralise the venom in a given case is difficult, because there is no clear end-point against which to titrate antivenom. The two brown snakes in Western Australia — the dugite (Pseudonaja affinis) and the western brown snake or gwardar (P. nuchalis) (Box 1) — both produce defibrination coagulopathy (in severe cases, afibrinogenaemia), but few other clinical features. Unlike the common or eastern brown snake (P. textilis), they rarely cause neurotoxicity in humans. Patients envenomed by brown snakes in Western Australia are often asymptomatic. Even when venom is neutralised by antivenom, there is a delay before fibrinogen is produced, so determining the dose required is difficult. An objective method for determining the neutralising dose based on ELISA measurement of venom concentrations in blood has been reported, but at present this is unavailable in Australia.4
Anecdotal experience, in vitro5 and animal work,6 as well as published case series,7,8 suggest that recommended doses may be insufficient for treating severe envenoming by brown snakes. As venom levels cannot be measured directly, current practice is to give an initial dose of antivenom and then observe fibrinogen levels and coagulation parameters in the 2–4 hours following administration.9 The return of detectable fibrinogen and improvement of coagulation parameters function as surrogate indicators of venom procoagulant neutralisation. Clinical experience suggests that, once circulating venom has been neutralised, the average time for regeneration of fibrinogen to clinically significant levels is less than 6 hours.9
The aim of this study was to describe the doses of antivenom administered in Western Australian teaching hospitals to adult patients with severe brown snake envenoming.
Retrospective chart review was undertaken by a trained investigator, blinded to the study question, using a preformatted data abstraction tool, in line with recommendations on the requirements for better-quality chart reviews.10 Charts from all three Western Australian adult tertiary referral hospitals that treat snake envenoming were examined for the period December 1991 to December 2001.
Charts were sourced using International Classification of Diseases (ICD)-9 and -10 external-cause coding to capture venomous and non-venomous snakebites (E905, W59, X20). We also checked pharmacy dispensing records (antivenom use) and abnormal results issued by the departments of haematology (coagulation parameters) and biochemistry (Venom Detection Kit results).
Inclusion criteria were (all of) presentation with possible snakebite; clinical syndrome consistent with brown snake envenoming; severe envenoming as defined below; and administration of CSL monovalent brown snake and/or polyvalent antivenom.
A clinical syndrome of brown snake envenoming was defined as coagulation parameters consistent with venom-induced defibrination coagulopathy (decreased serum fibrinogen, increased international normalised ratio [INR] and activated partial thromboplastin time [APTT]) without evidence of significant rhabdomyolysis or paralysis (consistent with tiger snake envenoming) with or without positive Venom Detection Kit (VDK) result for brown snake envenoming.
Severe brown snake envenoming was defined as the documentation of an undetectable fibrinogen level (< 0.3 g/L) during the clinical course. This fibrinogen cut-off level of 0.3 g/L was the lower limit of detection in the hospitals at the time. Neutralisation of circulating venom was defined as occurring when fibrinogen levels became detectable and remained detectable following the administration of antivenom. The dose of antivenom required to treat envenoming was defined as the number of ampoules administered before neutralisation of circulating venom.
Ethics committee approval was waived, as the study was registered as a quality improvement audit.
Two hundred and thirteen patient charts were identified and reviewed, of which 88 patients had either a positive VDK result for brown snake or clinical features consistent with brown snake envenoming. Thirty-five of these patients met inclusion criteria. Four other patients with brown snake envenoming and severe coagulopathy (INR > 10 and APTT > 180 s) were excluded from the study, as their fibrinogen levels were not measured.
In the study group of 35 patients, 23 (66%) were male, 18 (51%) had been bitten on the upper limb, 17 (49%) had been bitten within 50 km of central Perth, and 4 (11%) had been drinking alcohol at the time of envenoming.
The use of a pressure-immobilisation bandage was documented in the notes of 34 of the 35 patients; however, immediate application of a pressure-immobilisation bandage was documented for only six patients (17%).
Six patients received polyvalent antivenom first, four in rural hospitals. In addition to one ampoule of brown snake monovalent antivenom, one patient received a single ampoule of tiger snake monovalent antivenom (not included in total treating dose of antivenom analysis below).
The median time from the administration of the first dose of antivenom to return of detectable fibrinogen was 10 hours (range, 1.4–68 hours), and four patients (11%) had no detectable fibrinogen for more than 24 hours after the first dose of antivenom. From administration of the last dose of antivenom, the median time to return of detectable fibrinogen was 2.5 hours (range, 0.7–30 hours). All patients received exogenous clotting factors (fresh frozen plasma and/or cryoprecipitate). The mean dose was 10 units (range, 2–45 units).
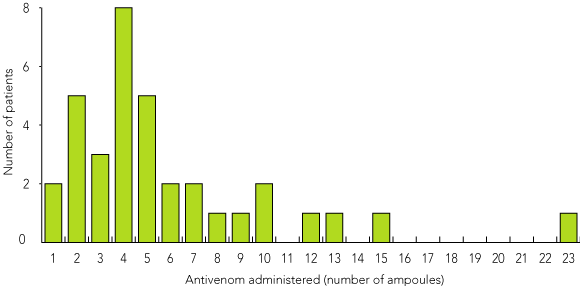
Box 2 shows the number of ampoules of antivenom received before return of detectable fibrinogen against the numbers of patients who received that dose. The dose of antivenom given before venom neutralisation ranged from one to 23 ampoules. After five ampoules of antivenom, venom neutralisation was achieved in two-thirds of envenomed patients (66%), and after 10 ampoules, 89%. Ten patients received further doses of antivenom after the return of detectable fibrinogen levels.
Two patients in the study died: a 72-year-old woman (previously reported11) and a 33-year-old man. Both were afibrinogenaemic when they had an intracerebral event. A third patient, a 31-year-old man (previously reported11), died of intracerebral haemorrhage, but was not included in the study because his fibrinogen levels were not recorded. Another patient, a 26-year-old man, had significant haemorrhaging from the site of a recent tooth extraction during the 30 hours he remained afibrinogenaemic. Four patients (11%) were documented as having renal impairment following envenoming.
From 1991 to 2001, severe brown snake envenoming was an uncommon presentation to Perth tertiary adult hospitals, but was associated with appreciable morbidity and mortality. Most patients received initial doses of antivenom that were too small to neutralise circulating venom and remained afibrinogenaemic and coagulopathic for prolonged periods. The median time from the first dose of antivenom until return of detectable fibrinogen was 10 hours, and in 11% of cases the duration of afibrinogenaemia exceeded 24 hours, reaching 68 hours for one patient. The grave risks associated with persistent afibrinogenaemia were demonstrated by the bleeding complications and deaths.
Our study suggests that larger doses of antivenom are required than previously recommended for brown snake envenoming. This is supported by previous in vitro,5 animal6 and clinical7,8 studies. Determining the appropriate initial dose in afibrinogenaemic patients is difficult in the absence of definitive measurement of free circulating venom levels after antivenom treatment. This study showed that two-thirds of patients would have been adequately treated with a single dose of five ampoules, and nearly 90% with 10 ampoules. Although it might be argued that antivenom was still being given to some of these patients after venom had been neutralised and fibrinogen had simply not yet been detected, in practice we believe this occurs uncommonly. We documented that, typically, these patients spend days in hospital with delays to further doses of antivenom, and delays in performing blood tests. Nevertheless, the doses we report as achieving venom neutralisation may overestimate the neutralising dose.
When choosing a starting dose, the grave risks of persistent afibrinogenaemia from undertreatment need to be balanced against the risk of allergic reactions and expense from too much antivenom. Monovalent brown snake antivenom has the smallest volume and is the cheapest of the monovalent antivenoms. Ten ampoules are roughly equivalent in volume (and therefore antigen load) and cost to one ampoule of polyvalent antivenom, a dose associated with a low incidence of allergic reactions. Until venom levels can be used clinically to determine antivenom doses, we feel that there is a compelling case for an initial dose of 10 ampoules of brown snake antivenom in Western Australia. We propose that this avoids the common situation of persistent afibrinogenaemia with its attendant significant risks with only a very small increase in risk of allergic reaction.
This study has several limitations. Although this is the largest series of brown snake envenomings reported, it is a chart review and involved relatively small numbers of patients. Small initial doses of monovalent and polyvalent antivenom may have been used in rural areas because of limited antivenom supplies. As we only studied patients admitted to Perth teaching hospitals, there may be referral bias. There may have been variations in practice and we were unable to analyse the influence of factors such as the use of exogenous coagulation factors, sampling times for coagulation studies and clinical end-points used to determine when circulating venom was neutralised. The study only involved patients with severe envenoming treated in Western Australian adult teaching hospitals, so the results may not be valid in other settings, and may not be applicable to children, although, in general, children develop a similar syndrome and require the same amount of antivenom as adults.
We note that patients required large doses of antivenom and remained afibrinogenaemic for prolonged periods despite apparently vigorous clotting-factor replacement therapy. A single controlled animal study showed that administration of fresh frozen plasma was commonly associated with persistent afibrinogenaemia.12 We hypothesise that clotting factors should not be necessary if a neutralising dose of antivenom is administered, unless there is active haemorrhaging or unusually slow hepatic repletion of clotting factor. This is consistent with current recommendations,1,9 but requires further, controlled studies.
Larger doses of brown snake antivenom than currently recommended are required for treatment of severe brown snake envenoming with afibrinogenaemia in Western Australia. Appropriate prospective studies measuring serum venom levels are needed to determine the exact dose. Until such data are available, the authors, who are responsible for advising on treatment for virtually all brown snake bites in Western Australia through the WA Poisons Information Centre, now routinely use a first dose of 10 ampoules of antivenom, followed by further doses of five ampoules if there is persistent afibrinogenaemia.
- 1. White J. Antivenom handbook. Melbourne: CSL Ltd, 2001.
- 2. Australian Venom Research Unit. Notes on the medical management of Australian venomous snake bites. Available at: www.avru.unimelb. edu.au/avruweb/doctors.htm (accessed Dec 2003).
- 3. Product information. Brown snake antivenom. Melbourne: CSL Ltd, 2000.
- 4. Theakston RD. An objective approach to antivenom therapy and assessment of first-aid measures in snake bite. Ann Trop Med Parasitol 1997; 91: 857-865.
- 5. Sprivulis PC, Jelinek GA, Marshall L. Efficacy and potency of antivenoms in neutralising the procoagulant effects of Australian snake venoms in dog and human plasma. Anaesth Intensive Care 1996; 24: 379-381.
- 6. Tibballs J, Sutherland SK. The efficacy of antivenom in prevention of cardiovascular depression and coagulopathy induced by brown snake (Pseudonaja) species venom. Anaesth Intensive Care 1991; 19: 530-534.
- 7. Barrett RL, Little M. Five years of snake envenoming in far north Queensland. Emerg Med 2003; 15: 500-510.
- 8. Jelinek GA, Hamilton T, Hirsch RL. Admissions for suspected snake bite to the Perth adult teaching hospitals, 1979 to 1988. Med J Aust 1991; 155: 761-764.
- 9. White J. Management of brown snake envenoming. Crit Care Resus 2002; 4: 81-86.
- 10. Gilbert EH, Lowenstein SR, Koziol-McLain J, et al. Chart reviews in emergency medicine research: where are the methods? Ann Emerg Med 1996; 27: 305-308.
- 11. Sprivulis PC, Jelinek GA. Fatal intracranial haematomas in two patients with brown snake envenomation. Med J Aust 1995; 162: 215-216.
- 12. Jelinek GA, Smith A, Lynch D, et al. The effect of adjunctive fresh frozen plasma administration on coagulation parameters and survival in a canine model of antivenom-treated Brown snake envenoming. Anaesth Intensive Care 2004. In press.





Abstract
Objective: To investigate the doses of antivenom administered to adult patients with severe brown snake envenoming.
Design and setting: Review of charts from Western Australian adult teaching hospitals, December 1991 to December 2001.
Patients: 35 patients with severe brown snake envenoming, defined prospectively as afibrinogenaemia (< 0.3 g/L) after a bite by a brown snake (genus Pseudonaja).
Main outcome measure: The dose of antivenom required to neutralise venom, defined prospectively as the dose of antivenom given before the return of detectable fibrinogen levels.
Results: Of 88 patients with brown snake envenoming admitted over the 10 years, at least 35 had severe envenoming. Afibrinogenaemia persisted for 10 hours (range, 1.4–68 hours) after the first dose of antivenom; in four patients afibrinogenaemia lasted more than 24 hours. The dose of antivenom given before venom neutralisation ranged from one to 23 ampoules. In two-thirds of cases, venom was neutralised with five ampoules, and 89% had venom neutralised with 10 ampoules. Two patients died, and another had serious bleeding complications. Another patient died during the study period from intracerebral haemorrhage, but did not have fibrinogen levels measured.
Conclusions: Patients received initial doses of antivenom too small to neutralise circulating venom, and remained afibrinogenaemic for prolonged periods, with serious consequences. The authors now use 10 ampoules as an initial dose in severe brown snake envenoming.