The red-bellied black snake (RBBS; Pseudechis porphyriacus) is a frequent cause of snake envenoming in south-eastern Australia. It inhabits most of the coastal and mountainous regions of eastern Australia, extending into parts of South Australia.1 The RBBS is an “elapid” snake (member of the family Elapidae) with an average length of 1.25 metres, but may be up to 2 metres long. It has a shiny black dorsal surface, red or pink lateral scales, and an underside that is cream, pink or red (Box 1).2
There is limited information on the clinical effects of RBBS envenoming3-5 or RBBS venom.6-11 In 1912, it was reported that the RBBS envenoming syndrome does not produce systemic coagulopathy or neuropathic effects.3 The few small series of cases reported since support this, with only one report of myotoxicity and only one report of coagulopathy.3-5,12 RBBS envenoming has therefore been regarded as not severe and “only cases with major systemic envenoming should be considered for antivenom therapy”.13 Initial reports to the Australian Snakebite Project (ASP) suggested that, in at least some cases, more significant systemic effects occurred. Tiger snake antivenom (TSAV) is recommended for RBBS envenoming, although black snake antivenom (BlSAV) can also be administered.13
We describe a cohort of patients from the ASP who had definite RBBS bites. The ASP is an ongoing, multicentre prospective observational study which recruits patients with suspected snakebite or snake envenoming from over 100 hospitals throughout Australia. The design of this study has previously been described in detail,14,15 and approval was obtained from 19 human research and ethics committees covering all institutions involved.
A search of the ASP database from January 2002 to June 2010 was performed. Definite RBBS bites were defined either as expert identification of the snake or as patient or non-expert identification of the snake plus a positive result in the black snake well of the snake venom detection kit (SVDK). Data extracted from the ASP database included age, sex, geographical location, local or systemic clinical effects, laboratory results, antivenom administration, and other treatments (eg, administration of antiemetics or analgesics). Clinical systemic envenoming syndromes were:
Venom concentrations were measured using enzyme immunoassay (EIA) for patients for whom serum samples were available. The methods are described in detail elsewhere.16,17 In brief, polyclonal antibodies (IgG) to RBBS were raised in rabbits, conjugated to biotin, and used to develop a sandwich EIA with streptavidin-horseradish peroxidase as the detecting agent. The limit of detection of the EIAs was 0.1 ng/mL. These assays only detect free venom that is not bound to antivenom. The peak venom concentration was recorded for each patient.
For data that are not normally distributed, medians, ranges and interquartile ranges (IQRs) are reported. Proportions are presented with 95% confidence intervals calculated using the Wilson’s procedure with a continuity correction.18 Statistical and graphical analyses were done in GraphPad Prism version 5.03 for Windows (GraphPad Software, San Diego, Calif, USA).
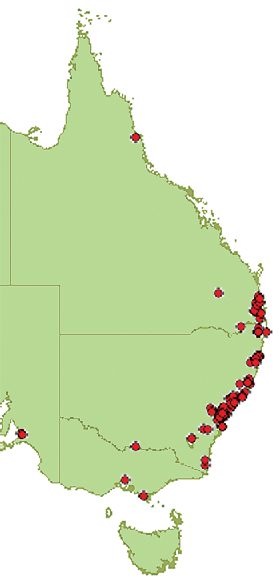
We identified 85 potential cases of RBBS bites from 873 snakebites in the ASP database for the period January 2002 to June 2010. Insufficient data were available for four cases. Of the remaining 81 patients with definite RBBS bites, 57 patients (70%) were systemically envenomed, one had local envenoming alone and 23 had no evidence of local or systemic envenoming. Most cases occurred in eastern New South Wales and south-eastern Queensland, two were from Victoria, one from South Australia and one from the Australian Capital Territory (Box 2). The median age of patients with definite RBBS bites was 36 years (IQR, 26–58 years). There were 10 children (12%) aged under 16 years and 58 male patients (72%). Box 3 provides details of the patient demographics and circumstances of the definite RBBS bites.
Local envenoming occurred in 55 of the 57 systemically envenomed patients (96%; 95% CI, 87%–99%) and in one patient without systemic envenoming, who had swelling and pain requiring 6 hours of analgesia. Local ulceration occurred in three systemically envenomed patients (5%; 95% CI, 1%–16%).
Systemic envenoming was characterised by systemic symptoms in 54 of 57 patients (95%; 95% CI, 84%–99%), anticoagulant coagulopathy with a raised aPTT in 35 patients (61%; 95% CI, 48%–74%) and myotoxicity in seven patients (12%; 95% CI, 5%–24%). There were no cases of clinically significant bleeding associated with the coagulopathy, and no cases of venom-induced consumption coagulopathy, neurotoxicity or thrombotic microangiopathy.
Of the seven patients with myotoxicity, all developed generalised myalgia and one developed muscle weakness, but ptosis or descending paralysis did not occur in any of these patients. Two other patients had a peak CK level > 1000 U/L (1779 U/L and 2723 U/L) without myalgia or muscle tenderness. Patients with myotoxicity had a longer median length of hospital stay than those systemically envenomed without myotoxicity (3 days v 21 h), and two of the seven with myotoxicity required intensive care unit admission. A 75-year-old woman with a peak CK level of 46 900 U/L required non-invasive ventilation for 24 hours for severe myotoxicity that resulted in bulbar and intercostal muscle weakness which was complicated by pneumonia. A 66-year-old man with a peak CK level of 56 000 U/L had a creatinine level of 151 μmol/L on admission (90 minutes after bite) that peaked at 210 μmol/L (60 hours after bite), but he did not develop anuria or other evidence of renal impairment. There were no deaths. Anosmia was reported in one patient, but this frequency may be an underestimate because follow-up of patients after discharge was rarely possible.
Results from SVDK testing of bite-site swabs were reported for 63 patients, and results from SVDK testing of urine were reported for five patients (four of whom had bite-site swab SVDK results). In 43 envenomed patients with SVDK results, 42 (98%; 95% CI, 86%–100%) had a positive result: 32 positive in the black snake well only (74%; 95% CI, 59%–86%), eight positive in black snake and tiger snake wells (19%; 95% CI, 9%–34%) and two in the tiger snake well only (5%; 95% CI, 1%–17%). Of the 19 non-envenomed patients for whom bite-site SVDK testing was performed, 15 (79%; 95% CI, 54%–93%) had positive results.
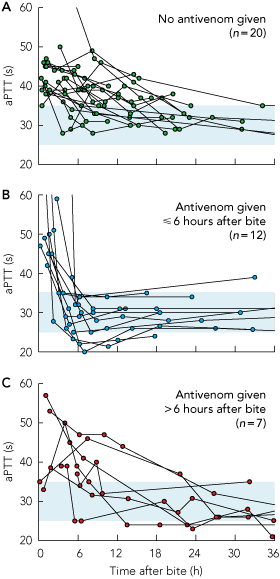
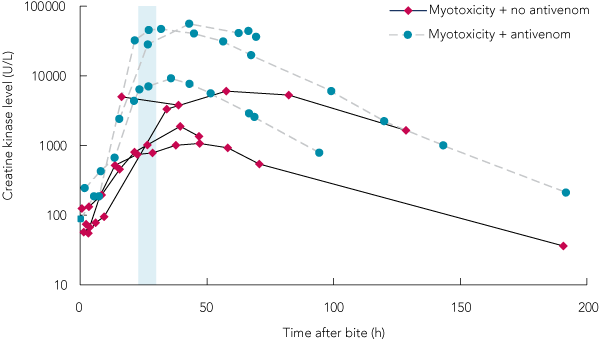
Box 4 shows the time course of the aPTT for patients given early antivenom (≤ 6 h after bite), delayed antivenom (> 6 h after bite) and those not given antivenom. Box 5 shows the time course of the CK levels in patients with myotoxicity, three of whom were given antivenom and four of whom were not given antivenom. Five of the seven patients with myotoxicity and both patients with a CK > 1000 U/L and no myalgia had an early raised aPTT.
Antivenom was administered in 22 of the 57 systemically envenomed patients (39%; 95% CI, 26%–52%). Thirteen received one vial of TSAV, four received two vials of TSAV, four received one vial of BlSAV (one of whom was given half the vial only owing to an adverse reaction) and one received one vial of polyvalent antivenom. Eight of these 22 patients had an immediate hypersensitivity reaction to the antivenom; seven had mild reactions but one had anaphylaxis with hypotension. The frequency of reactions was similar for TSAV (06/17) and BlSAV (2/4).
The aPTT appeared to normalise rapidly with antivenom therapy. Ten patients with a raised aPTT who were given antivenom ≤ 6 hours after the bite had a normal aPTT when blood was next collected (Box 4, Box 6). Of seven patients with a raised aPTT who received antivenom more than 6 hours after the bite, the aPTT had normalised before antivenom was given in five (20–32 h after bite) and aPTT recovered by the time of the next blood collection in the other two (8.8 h and 9 h after bite). Raised CK level did not appear to resolve more rapidly when antivenom was administered (Box 5). Three patients with myotoxicity received antivenom but were given it 23, 26 and 30 hours after the bite, respectively. The three patients with local ulceration were given antivenom at 2.25, 26 and 30 hours after the bite, respectively, and ulcers were not noted until more than 24 hours after the bite. Box 6 shows the clinical features of patients with anticoagulant coagulopathy who were given early antivenom (≤ 6 h after bite) and patients with anticoagulant coagulopathy who were given delayed antivenom (> 6 h after bite) or no antivenom. Myotoxicity did not occur in any patients given early antivenom but occurred in 20% of patients given no antivenom or delayed antivenom.
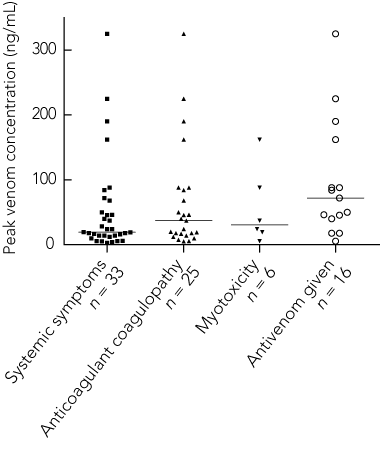
Serum samples from 47 patients were available for testing. Venom was not detected in all eight non-envenomed patients for whom samples were available. Of the remaining 39 (who were all systemically envenomed), 22 did not receive antivenom, 15 of those who received antivenom had post-antivenom samples, and two of those who received antivenom had post-antivenom samples only. The median peak RBBS venom concentration from the 37 systemically envenomed patients with more than one serum sample available was 19 ng/mL (IQR, 12–50 ng/mL; range, 3–360 ng/mL), which did not correlate with clinical severity (Box 7). The envenomed patient with a negative SVDK test result had a peak venom concentration of 50 ng/mL, and the two envenomed patients with a positive SVDK result for tiger snake had peak venom concentrations of 12 ng/mL and 72 ng/mL.
Higher venom concentrations were associated with antivenom administration but not with major clinical effects (Box 7). In the 17 patients given antivenom who had venom concentration measured, no venom was detected in any blood sample taken after antivenom was given. Of these 17 patients, nine received one vial of TSAV, four received two vials of TSAV and four received at least one vial of BlSAV.
This study has confirmed that RBBS envenoming causes both local and systemic symptoms, as previously noted. However, in contrast to previous studies, it shows that the majority of RBBS envenoming cases develop an anticoagulant coagulopathy characterised by a raised aPTT, and that a small proportion of patients develop significant myotoxicity. The results suggest that the coagulopathy develops within hours, resolves over about 24 hours in untreated patients, is not clinically significant and is rapidly reversed with antivenom administration. In contrast, myotoxicity develops slowly and may persist for up to 7 days. It did not develop in anyone who received early antivenom, and did not appear to reverse with antivenom administration. There was poor correlation between peak venom concentrations and the severity of clinical envenoming syndromes, but venom was only detected in the serum of patients with systemic envenoming. One vial of either TSAV or BlSAV was sufficient to bind all venom. Immediate-type hypersensitivity reactions occurred in over a third of antivenom administrations, demonstrating that antivenom administration is associated with significant adverse effects.
Previously, RBBS was thought to cause only minor effects but this was based on a limited number of cases. In two small series of 10 and 15 definite cases of RBBS envenoming in South Australia,4,5 there were 14 cases of systemic envenoming, but only one patient had coagulopathy and the remainder only had systemic symptoms.4,5 A further five cases again reported only local and systemic symptoms.3 In contrast to previous studies, we have shown that an anticoagulant coagulopathy occurred in the majority (61%) of envenomed patients. However, no patients developed life-threatening haemorrhage, which could explain why such a coagulopathy has not been previously recognised.
Other clinical effects have been reported with RBBS envenoming including anosmia,3,12 which has also been reported for other black snake species.19 Anosmia was only reported in one patient in our study, but patients may only become aware of it after hospital discharge, making it easily missed in the acute care setting. Local envenoming occurred in almost all cases of RBBS envenoming. Pain relief was required in about half of envenomed patients, supporting previous reports that local effects are an important part of this syndrome.3
Studies of RBBS venom explain the clinical effects we observed. It is surprising that the coagulopathy has been under-recognised in humans. The coagulant effects of the venom were first recognised over 100 years ago, when coagulopathy was demonstrated in dogs injected with venom.20 A prothrombin activator toxin has been identified in the venom, but differs to those in other Australasian elapids and its clinical relevance is unclear.21,22 Although an anticoagulant toxin has not been isolated, the venom has anticoagulant activity based on in vitro clotting studies.8 The myotoxic effects of RBBS venom have been demonstrated in vitro and showed that both TSAV or BlSAV were able to prevent but not reverse this effect.10
TSAV is currently recommended for RBBS envenoming,13 which is reflected in it being the most frequently used antivenom in our study. It is believed that TSAV is more appropriate than BlSAV because it is cheaper and a lower volume is required. Our study showed that one vial of TSAV is likely to be sufficient to bind all venom, with venom undetectable in all tested patients after administration of one vial of antivenom. It would appear that the current recommended dose and type of antivenom is appropriate for RBBS envenoming, although it possibly should be administered earlier and more often than it is in current practice. However, we did observe high rates of hypersensitivity reactions to both TSAV and BlSAV, which need to be balanced against benefits.
|
|
|
* The shaded area indicates the timing of antivenom in those who received it (23, 26 and 30 h after bite). |
|
Delayed or no antivenom (n = 25) |
|||||||||||||||
|
|
|||||||||||||||
|
Serum creatine kinase level > 1000 U/L |
|||||||||||||||
|
Peak serum creatine kinase level (U/L)‡ |
|||||||||||||||
|
Peak venom concentration (ng/mL)‡ |
|||||||||||||||
|
|
|||||||||||||||
7 Peak RBBS venom concentrations in patients with major clinical effects and those given antivenom*
|
|
|
* Horizontal lines represent median values. RBBS = red-bellied blacked snake. |
- Andrew Churchman1
- Margaret A O’Leary2
- Nicholas A Buckley3
- Colin B Page1,2
- Alan Tankel4
- Chris Gavaghan5
- Anna Holdgate6,7
- Simon G A Brown8,9
- Geoffrey K Isbister10,2
- 1 Emergency Department, Princess Alexandra Hospital, Brisbane, QLD.
- 2 Department of Clinical Toxicology and Pharmacology, Calvary Mater Newcastle, Newcastle, NSW.
- 3 Medical Professorial Unit, Prince of Wales Hospital Medical School, University of New South Wales, Sydney, NSW.
- 4 Emergency Department, Coffs Harbour Base Hospital, Coffs Harbour, NSW.
- 5 Emergency Department, Lismore Base Hospital, Lismore, NSW.
- 6 Emergency Department, Liverpool Hospital, Sydney, NSW.
- 7 Department of Anaesthetics, Emergency Medicine and Intensive Care, University of New South Wales, Sydney, NSW.
- 8 Centre for Clinical Research in Emergency Medicine, Western Australian Institute for Medical Research, Perth, WA.
- 9 Emergency Medicine, Royal Perth Hospital, University of Western Australia, Perth, WA.
- 10 Discipline of Clinical Pharmacology, University of Newcastle, Newcastle, NSW.
We thank the ASP clinical investigators who recruited patients to the study — Richard Whitaker and Lambros Halkidis (Cairns Base Hospital), David Spain and Graham Ireland (Gold Coast Hospital), Mark Miller (John Hunter Hospital), Andis Graudins (Prince of Wales Hospital), Naren Gunja (Westmead Hospital) — and the ASP laboratory investigators. We also acknowledge the many referrals from the poison information centres and clinical toxicologists, and thank the many other nurses, doctors and laboratory staff who helped recruit patients and collect samples.
None identified.
- 1. Shine R. Intraspecies variation in thermoregulation, movements and habitat use by Australian blacksnakes, Pseudechis porphyriacus (Elapidae). J Herpetol 1987; 21: 165.
- 2. Cogger HG. Reptiles and amphibians of Australia. 6th ed. Port Melbourne: Reed Books Australia, 2000.
- 3. Pearn J, McGuire B, McGuire L. The envenomation syndrome caused by the Australian red-bellied black snake Pseudechis porphyriacus. Toxicon 2000; 38: 1715-1729.
- 4. White J. A review of 105 cases of suspected snakebite in South Australia. In: Gopalakrishnakone P, Tan C, editors. Progress in venom and toxin research. Singapore: National University of Singapore, 1987: 15-19.
- 5. White J. A review of snakebites and suspected snakebites treated in South Australia with particular reference to snake handlers. Recent Adv Toxin Res 1991: 366-377.
- 6. Doery HM, Pearson JE. Haemolysins in venoms of Australian snakes. Observations on the haemolysins of the venoms of some Australian snakes and the separation of phospholipase A from the venom of Pseudechis porphyriacus. Biochem J 1961; 78: 820-827.
- 7. Schmidt JJ, Middlebrook JL. Purification, sequencing and characterization of pseudexin phospholipases A2 from Pseudechis porphyriacus (Australian red-bellied black snake). Toxicon 1989; 27: 805-818.
- 8. Vaughan GT, Sculley TB, Tirrell R. Isolation of a hemolytic, toxic phospholipase from the venom of the Australian red-bellied black snake (Pseudechis porphyriacus). Toxicon 1981; 19: 95-101.
- 9. Kellaway CH. Observations of the certainly lethal dose of the black snake (Pseudechis porphyriacus) for the common laboratory animals. Med J Aust 1930; 2: 33-41.
- 10. Ramasamy S, Isbister GK, Hodgson WC. The efficacy of two antivenoms against the in vitro myotoxic effects of black snake (Pseudechis) venoms in the chick biventer cervicis nerve-muscle preparation. Toxicon 2004; 44: 837-845.
- 11. Ramasamy S, Fry BG, Hodgson WC. Neurotoxic effects of venoms from seven species of Australasian black snakes (Pseudechis): efficacy of black and tiger snake antivenoms. Clin Exp Pharmacol Physiol 2005; 32: 7-12.
- 12. Sutherland SK, Tibballs J. Genus Pseudechis, black snakes. Australian animal toxins. Melbourne: Oxford University Press, 2001: 137-159.
- 13. White J. CSL antivenom handbook. 2nd ed. Melbourne: CSL, 2001.
- 14. Isbister GK, Brown SG, MacDonald E, et al. Current use of Australian snake antivenoms and frequency of immediate-type hypersensitivity reactions and anaphylaxis. Med J Aust 2008; 188: 473-476. <MJA full text>
- 15. Gan M, O’Leary MA, Brown SG, et al. Envenoming by the rough-scaled snake (Tropidechis carinatus): a series of confirmed cases. Med J Aust 2009; 191: 183-186. <MJA full text>
- 16. Kulawickrama S, O’Leary MA, Hodgson WC, et al. Development of a sensitive enzyme immunoassay for measuring taipan venom in serum. Toxicon 2010; 55: 1510-1518.
- 17. O’Leary MA, Isbister GK, Schneider JJ, et al. Enzyme immunoassays in brown snake (Pseudonaja spp.) envenoming: detecting venom, antivenom and venom-antivenom complexes. Toxicon 2006; 48: 4-11.
- 18. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17: 857-872.
- 19. Isbister GK, Hooper MR, Dowsett R, et al. Collett’s snake (Pseudechis colletti) envenoming in snake handlers. QJM 2006; 99: 109-115.
- 20. Martin CJ. On some effects upon the blood produced by the injection of the venom of the Australian black snake (Pseudechis porphyriacus). J Physiol 1893; 15: 380-400.
- 21. Chester A, Crawford GP. In vitro coagulant properties of venoms from Australian snakes. Toxicon 1982; 20: 501-504.
- 22. St Pierre L, Masci PP, Filippovich I, et al. Comparative analysis of prothrombin activators from the venom of Australian elapids. Mol Biol Evol 2005; 22: 1853-1864.






Abstract
Objective: To describe the clinical features and laboratory findings in patients with definite red-bellied black snake (RBBS; Pseudechis porphyriacus) bites, including correlation with results of venom assays.
Design, patients and setting: Prospective cohort study of patients with definite RBBS bites, recruited to the Australian Snakebite Project from January 2002 to June 2010.
Main outcome measures: Clinical and laboratory features of envenoming; peak venom concentrations and antivenom treatment.
Results: There were 81 definite RBBS bites; systemic envenoming occurred in 57 patients (70%) and local envenoming alone occurred in one patient. Systemic envenoming was characterised by local envenoming in 55 patients (96%), systemic symptoms in 54 patients (95%), anticoagulant coagulopathy with a raised activated partial thromboplastin time (aPTT) in 35 patients (61%) and myotoxicity in seven patients (12%). One patient required non-invasive ventilation for severe myotoxicity that resulted in muscle weakness. Three patients developed local ulceration. There were no deaths. Twenty-two envenomed patients (39%) received tiger snake or black snake antivenom, and administration within 6 hours of the bite was associated with normalisation of the aPTT. Eight patients (36%) had immediate hypersensitivity reactions to antivenom, including one case of anaphylaxis. The median peak venom concentration in 37 systemically envenomed patients with serum available was 19 ng/mL (interquartile range, 12–50 ng/mL; range, 3–360 ng/mL), which did not correlate with clinical severity. In 17 patients who received antivenom and had venom concentration measured, no venom was detected in serum after the first antivenom dose, including nine who were given one vial of tiger snake antivenom.
Conclusion: RBBS envenoming caused local effects, systemic symptoms, anticoagulant coagulopathy and, uncommonly, myotoxicity. One vial of tiger snake or black snake antivenom appears to be sufficient to remove venom and neutralise reversible effects, but hypersensitivity reactions occurred in over a third of patients.