Snakebite remains an important cause of morbidity in tropical Australia, despite the declining national snakebite rates.1 Prospective studies are required in Australia to better define epidemiological and clinical features of snakebite from the many Australasian elapid (front-fanged venomous land snake) species.
The current study was begun in September 1989 to evaluate snakebite in the tropical “Top End” of the Northern Territory. Its aim was to describe the local epidemiology and the envenoming syndromes from the various venomous snakes present in the region. The Top End has a climate divided into the hot monsoonal rainy season from November to May and the cooler mid-year dry season, but snakes remain active year-round.
From September 1989, a standard data collection form has been used for patients presenting to RDH with suspected snakebite. Variables listed include demographic details, nature of the suspected bite, first aid treatment, and clinical parameters reflecting the envenoming syndromes of Australian snakes. Envenomed patients are managed by RDH emergency department and inpatient staff in consultation with the author, according to local protocols.2,3 Data relating to antivenom use, clinical features and response to antivenom were also recorded prospectively.
Each case is only recorded as a definite snakebite if there is a clear description of a bite from a snake that was seen, or if there is clinical evidence of snakebite envenoming. Otherwise, the case is recorded as “uncertain”. For bitten patients, envenoming is categorised according to presence of regional lymphadenitis (differential tenderness on palpation), non-specific systemic features (nausea, vomiting, abdominal pain and headache), coagulopathy (confirmed by prolonged prothrombin time and/or partial thromboplastin time), neurotoxicity (defined as at least a finding of definite ptosis) and myotoxicity (confirmed by blood creatine kinase [CK] concentration higher than 500 U/L; normal range, < 220 U/L).
Systemic envenoming is defined as definite snakebite and at least one of coagulopathy, neurotoxicity, myotoxicity, or non-specific systemic features of envenoming.
Positive identification of the snake is only recorded where the snake that definitely bit the patient is brought in (dead or alive) for identification, either by the author using standard identification keys4,5 or by a qualified expert (see Acknowledgements), or if there is a positive Venom Detection Kit (VDK; CSL Ltd, Parkville, VIC) result in a patient whose clinical features are consistent with the test result. Because in the Top End the only species of the brown snake (Pseudonaja) and black snake (Pseudechis) genera are the western brown (Pseudonaja nuchalis) and mulga (Pseudechis australis) snakes,6 positive results of the genus-specific VDK are assumed to be these species. Although death adders in the Top End had previously all been considered the northern death adder species (Acanthophis praelongus), there are ongoing revisions of the genus Acanthophis, so these are identified only as Acanthophis spp. in this study.5
All patients presenting to RDH with suspected snakebite from 1 September 1989 to 31 March 1998 were included in an epidemiological assessment of all suspected snakebites. Subsequently, from 31 March 1998 until 31 March 2003, data have only been routinely collected for confirmed snakebites from positively identified snakes.
For the epidemiological study, data were stored in an Epi-Info database and descriptive statistics, risk ratios, incidence rates and incidence rate ratios and their 95% confidence intervals were calculated using Epi-Info.7 Population figures used were from Territory Health Services distribution and projection estimates from the Australian Bureau of Statistics 1991 Census data, with averages of the 1993 and 1994 figures (epidemiological study mid-point) used as denominators for incidence rates. Denominators for incidence rates were based on the population using RDH as the primary referral centre; patients transferred to RDH from the two Top End regional hospitals were excluded from calculations of incidence rates. For incidence rates, the total case numbers were divided by 8.58, reflecting the 8 years and 7 months of the epidemiological study.
Associations between categorical variables were analysed using the χ2 test with Yates’ correction or Fisher’s exact test, as appropriate. Comparisons using ages of patients and time of day of bite were made using the two-sample t test and Wilcoxon two-sample test, respectively. P < 0.05 was considered significant.
There were 348 suspected snakebites, with 216 patients (62%) definitely bitten (see Box 1). Of those definitely bitten, ages ranged from 1 to 75 years (mean, 30 years; SD, 16 years), 150 (69%) were male and 61 (28%) were Aboriginal.Box 2 shows the clinical features in the 65 systemically envenomed patients.Box 3 shows the incidence rates for definite snakebite and for envenoming.
Air evacuation from rural NT areas to RDH was required for 112 patients, three by helicopter and the rest by the NT Aerial Medical Services, at an average cost of about $3000 per patient. Two other patients were evacuated to RDH by plane from the adjacent Kimberley region of Western Australia.
Seventy-eight children under 15 years of age had suspected bites, and 47 were definitely bitten (22% of both groups). Fifteen children were clinically envenomed (12 with systemic features and three with only lymphadenitis). In two children, the diagnosis of snake envenoming was delayed because no history of snakebite was forthcoming on initial presentation.
Snakebite occurred throughout the year, with the highest incidence during March to May, accounting for 36% of definite bites, whereas only 9% occurred in June–July. The median time of definite bites was 1530, and 78 patients (36%) were bitten at night (1900 to 0600).
Of those definitely bitten, 127 (59%) were bitten on the foot or ankle, 53 (25%) on the hand or wrist, 21 (10%) on the lower leg, 2 (1%) on the forearm and 13 (6%) elsewhere or uncertain as to where. Of those bitten on the hand, 16 (30%) were intoxicated by alcohol at the time of bite, compared with 12 (7%) of those bitten elsewhere (χ2 = 16.5; P < 0.0001). In comparison with non-Aboriginal people, Aboriginal people were one-third as likely to be bitten on the hand as elsewhere (RR, 0.32; 95% CI, 0.15–0.72). This is likely to reflect a greater understanding in Aboriginal people of the dangers of handling snakes, as well as their common lack of protective footwear.
Box 4 summarises the 156 snakebites for which the snake species was confirmed, and Box 5 shows the clinical features of the 74 bites from snakes in the four potentially lethal genera present in the Top End, highlighting the distinct envenoming syndromes from these snakes. There were no deaths over the 13.6 years.
This prospective study is the largest single series of suspected and confirmed snakebite reported from an Australian hospital. Snakebite incidence rates in Australia have previously been published from southeast Queensland, with incidences of snakebite in children of 7.1 per 100 000 children annually in 1971–19758 and 2.0–3.5 per 100 000 in 1978–1987,9 compared with 18.3 per 100 000 in children in this study from 1989–1998. Australasian elapid snakes are also present on the island of New Guinea, where the highest reported incidence rate of snakebite was 526 per 100 000 in the Papuan coastal Kairuku subprovince, north of Port Moresby.10 This is considerably higher than the rate of 61.4 per 100 000 for remote Top End Aboriginal communities in this study, and approaches the highest rates documented in the world.11,12
In this study, the envenoming rate was highest among Top End rural Aboriginal adult males (45.2 per 100 000), compared with 81.8 per 100 000 in rural central Papua.13 The incidence of envenoming in Top End children was 4.0 per 100 000, compared with 0.2 per 100 000 in a Queensland study from a decade earlier9 and 17.6 per 100 000 in children from the Port Moresby region.13
In addition to the Queensland snakebite studies,8,9 there have been retrospective series of adults14,15 and children16 from Western Australia, adults and children from South Australia17,18 and children from Victoria,19 and a prospective study of 11 cases from Sydney.20 Jelinek et al described 193 patients with suspected snakebite admitted to the three Perth adult teaching hospitals over a 10-year period from 1979.15 Of these, 53 were envenomed, including 13 of the 76 patients admitted to one Perth hospital.14 White described a series of 244 suspected snakebites in South Australia over a 20-year period from 1970, with 75 having evidence of systemic envenoming.18 The Top End study reported here, and a recent series from north Queensland,21 confirm earlier data showing a higher incidence of snakebite in tropical Australia than in temperate regions of Australia.22
As with all Australian studies, there was a male predominance among snakebite victims in the Top End, with males twice as likely as females to be bitten or envenomed. This is consistent with greater occupational and recreational exposure. However, the male predominance seen in children in the Queensland7,9 and Western Australian16 studies was not evident in the Top End.
In temperate Australia, snakebite is commonest in January and very unusual in winter, reflecting snake activity and hibernation.15 Snakes in tropical Australia are active all year and envenoming can occur in any month,2,13 although it is less common in the cooler months of June to August. March to May were the commonest months for bites in this study. This probably relates to increased human exposure from outdoor activity at the end of the monsoon as well as to snake behaviour.
In this study, 36% of those definitely bitten and 32% of those envenomed (25/79) were bitten at night (1900 to 0600 hours). This is higher than in the Papua New Guinea study of envenomed patients (15% at night)13 and the studies from Queensland (all bites, 13% and 9% at night)8,9 and Perth (all bites, 12.5% at night),15 all of which defined night as 1800 to 0600. The higher Top End night-time proportion of bites probably reflects relatively greater outdoor nocturnal behaviour of people, as well as continuing snake activity in the evenings, which, for example, is recognised for death adders, western brown and mulga snakes.4-6,23
The predominance of bites on the lower limbs is consistent with other studies, although the proportion on the ankle or foot (59%) was higher than in Queensland (42% and 41%)8,9 and Western Australian (49%)15 studies, but less than in Papua New Guinea.13 This relates to absence of sturdy footwear in many circumstances in the Top End and in Papua New Guinea. The association between intoxication and bites on the hands is not surprising and has been noted before.18
There were three documented cases in which the snakebite was clearly not a defensive response. Overambitious feeding attempts by the snakes (mulga snake, slatey grey snake, and olive python) may account for this. In each case, the victim woke at night to find a large snake chewing on an exposed extremity (two toes and one wrist) (Box 6).
Many descriptions of Australian envenoming syndromes are from cumulative case reports, although series of bites from various species have been very informative.14-17,24-27 In this study, strict definitions for envenoming parameters and for confirming snake identity allowed envenoming syndromes to be summarised for the three most common highly venomous Top End elapids (Box 5). Among bites from 29 less venomous elapids and five rear-fanged venomous colubrids (Box 4), there was no life-threatening envenoming, with only occasional, non-specific systemic features in bites from whip snakes (Demansia spp.) (data not shown).
Bite-site swelling, lymphadenitis and non-specific systemic features are most prominent in mulga snake envenoming and, importantly, may occasionally be absent in brown snake bite with severe consumptive coagulopathy and fibrinogen depletion (Box 7) and in death adder bite with neurotoxicity. Myotoxicity is the major feature of mulga snake envenoming, with severe cases having marked limb swelling, CK levels over 10 000 U/L and frank myoglobinuria (Box 8). Myotoxicity has generally not been considered important in death adder and brown snake envenoming, but elevated CK levels were occasionally seen in this study with bites from these snakes. A myotoxin has been recently described from one death adder species.28
The dramatic early collapse (within 30 minutes) with recovery, which was seen in more than half those with systemic envenoming from western brown snake bites, has been attributed to transient myocardial dysfunction and hypotension associated with microvascular thrombi during the early phase of the consumptive coagulopathy.2,29 Early collapse is also seen in taipan and tiger snake envenoming with coagulopathy,2 but neither early collapse nor coagulopathy was seen in any mulga snake or death adder envenomings in this study.
Neurotoxicity is the most important feature of death adder envenoming, but occurred in fewer than half the patients in this series. This may reflect selection bias towards more severe cases in previous series from Papua New Guinea,24,25 or the death adder species in the Top End may be less venomous. Although both mulga and brown snake venoms have neurotoxins,4-6,23 neurotoxicity was very uncommon in bites from these species. The rarity of neurotoxicity in envenoming from brown snakes despite the presence of potent presynaptic neurotoxins has been called the “brown snake paradox”.2
This prospective study has defined the epidemiological features of snakebite in the Top End and described the envenoming syndromes from the major venomous snakes in the region. Prospective studies are needed elsewhere in Australia to better define the epidemiology of snakebite and the clinical features of envenoming from the diverse species of elapids.
1 Features of 216 patients with definite snakebite: 1989–1998
* No signs or symptoms beyond bite site.
2 Clinical features in 65 systemically envenomed patients: 1989–1998
Bite-site swelling |
50 |
(77%) |
Regional lymphadenitis |
50 |
(77%) |
Early collapse (30 minutes) with loss of consciousness |
10 |
(15%) |
Non-specific systemic features |
||
Nausea |
47 |
(72%) |
Abdominal pain |
34 |
(52%) |
Headache |
32 |
(49%) |
Vomiting |
31 |
(48%) |
Coagulopathy |
27 |
(42%) |
Neurotoxicity |
13 |
(20%) |
Myotoxicity |
11 |
(17%) |
3 Incidence rates and incidence rate ratios for definite bites and envenoming: 1989–1998
|
Population estimate* |
Definite bites† |
Annual bites per 100 000 |
RR |
(95% CI) |
Number envenomed‡ |
Annual envenoming per 100 000 |
RR |
(95% CI) |
||||||
Overall |
100 845 |
201 |
23.2 |
|
|
66 |
7.6 |
|
|
||||||
Sex |
|
|
|
|
|
|
|
|
|
||||||
Male |
52 245 |
135 |
30.1 |
1.9 |
(1.4–2.6) |
46 |
10.3 |
2.1 |
(1.3–3.6) |
||||||
Female |
48 600 |
66 |
15.8 |
1.0 |
|
20 |
4.8 |
1.0 |
|
||||||
Age |
|
|
|
|
|
|
|
|
|
||||||
< 15 years |
26 162 |
41 |
18.3 |
0.7 |
(0.5–1.0) |
9 |
4.0 |
0.5 |
(0.2–0.9) |
||||||
≥ 15 years |
74 683 |
160 |
25.0 |
1.0 |
|
57 |
8.9 |
1.0 |
|
||||||
Children < 15 years |
|
|
|
|
|
|
|
|
|
||||||
Male |
13 329 |
22 |
19.2 |
1.1 |
(0.6–2.1) |
2 |
1.8 |
0.3 |
(0.1–1.3) |
||||||
Female |
12 833 |
19 |
17.3 |
1.0 |
|
7 |
6.4 |
1.0 |
|
||||||
Location |
|
|
|
|
|
|
|
|
|
||||||
Aboriginal community‡ |
8 917 |
47 |
61.4 |
3.2 |
(2.3–4.4) |
18 |
23.5 |
3.9 |
(2.3–6.6) |
||||||
Other§ |
91 928 |
154 |
19.5 |
1.0 |
|
48 |
6.1 |
1.0 |
|
||||||
Aboriginal community‡ |
|
|
|
|
|
|
|
|
|
||||||
Male ≥ 15 years |
2 837 |
22 |
90.4 |
1.7 |
(0.9–3.4) |
11 |
45.2 |
3.4 |
(1.0–12.2) |
||||||
Female ≥ 15 years |
2 638 |
12 |
53.0 |
1.0 |
|
3 |
13.3 |
1.0 |
|
||||||
* 1993–1994 average for Royal Darwin Hospital (RDH) primary catchment, excluding Gove and Katherine hospital catchments. † Excludes patients transferred to RDH from Gove, Katherine or Kimberley, WA. ‡ Aboriginal people living in remote communities. § All other people, predominantly Darwin urban and surrounding semi-rural populations. |
|||||||||||||||
4 Confirmed snake identification: 1989–2003
Common name |
Scientific name |
Snake brought |
VDK* positive |
Total |
|||||||||||
Elapids (venomous) |
|||||||||||||||
Highly venomous |
|||||||||||||||
Western brown snake |
Pseudonaja nuchalis |
12 |
19 |
31 |
|||||||||||
Mulga snake |
Pseudechis australis |
8 |
12 |
20 |
|||||||||||
Death adder |
Acanthophis spp. |
13 |
8 |
21 |
|||||||||||
Taipan† |
Oxyuranus scutellatus |
1 |
|
1 |
|||||||||||
Inland taipan† |
Oxyuranus microlepidotus |
1 |
|
1 |
|||||||||||
Blue-bellied black snake† |
Pseudechis guttatus |
1 |
|
1 |
|||||||||||
Less venomous |
|||||||||||||||
Black whip snakes |
Demansia spp.‡ |
17 |
|
17 |
|||||||||||
Marble-headed whip snake |
Demansia olivacea |
5 |
|
5 |
|||||||||||
Secretive snake |
Rhinoplocephalus pallidiceps |
4 |
|
4 |
|||||||||||
Moon snake |
Furina ornata |
3 |
|
3 |
|||||||||||
Venomous colubrids (rear-fanged) |
|||||||||||||||
Brown tree snake |
Boiga irregularis |
3 |
|
3 |
|||||||||||
White-bellied mangrove snake |
Fordonia leucobalia |
1 |
|
1 |
|||||||||||
Water snake |
Enhydris polylepis |
1 |
|
1 |
|||||||||||
Non-venomous |
|||||||||||||||
Slatey grey snake |
Stegonotus cucullatus |
10 |
|
10 |
|||||||||||
Children’s python |
Liasis childreni |
12 |
|
12 |
|||||||||||
Water python |
Liasis fuscus |
9 |
|
9 |
|||||||||||
Carpet python |
Morelia spilota variegata |
8 |
|
8 |
|||||||||||
Olive python |
Liasis olivacea |
4 |
|
4 |
|||||||||||
Common (golden) tree snake |
Dendrelaphis punctulatus |
2 |
|
2 |
|||||||||||
Keelback |
Tropidonophis mairii |
2 |
|
2 |
|||||||||||
* Venom Detection Kit test on urine or bite swab (performed if patient envenomed and no snake brought for identification). † Bites from captive snakes in private collection. ‡ Demansia atra or Demansia papuensis. |
|||||||||||||||
5 Clinical features of the four highly venomous Top End elapids: 1989–2003
|
Western brown (n = 31) |
Mulga (n = 20) |
Death adder (n = 21) |
Taipan (n = 2) |
|||||||||||
Bite-site swelling |
9 (29%) |
19 (95%) |
13 (62%) |
2 |
|||||||||||
Regional lymphadenitis |
19 (61%) |
16 (80%) |
13 (62%) |
1 |
|||||||||||
Systemic envenoming |
26 (84%) |
19 (95%) |
17 (81%) |
2 |
|||||||||||
Early collapse (within 30 min) |
14 (45%) |
0 |
0 |
0 |
|||||||||||
Non-specific systemic features* |
23 (74%) |
19 (95%) |
15 (71%) |
2 |
|||||||||||
Coagulopathy |
26 (84%) |
9 (45%) |
0 |
2 |
|||||||||||
Neurotoxicity |
4 (13%) |
3 (15%) |
8 (38%) |
1 |
|||||||||||
Myotoxicity |
3 (10%) |
12 (60%) |
1 (5%) |
1 |
|||||||||||
Prior intoxication |
9 (29%) |
7 (35%) |
2 (10%) |
1 |
|||||||||||
Antivenom given |
25 (81%) |
15 (75%) |
6 (29%) |
2 |
|||||||||||
* One or more of abdominal pain, nausea, vomiting or headache. |
|||||||||||||||
6 Predatory bite on the wrist of a sleeping 7-year-old
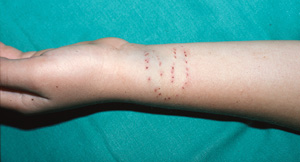
This semicircular row of teeth marks is from a large olive python.
- Bart J Currie1
- Tropical and Emerging Infectious Diseases Division, Menzies School of Health Research, Charles Darwin University, and Northern Territory Clinical School, Flinders University, Darwin, NT.
I am extremely grateful to the many medical, nursing and laboratory colleagues who have assisted with this study and with treating the patients, especially David Burgess, Jim Burrow, Geoff Isbister, Susan Jacups, Melita McKinnon, Didier Palmer, Vijay Selvarajah, Marg St Leone and Yvonne Wood. The herpetological expertise of Graeme Gow and Paul Horner has been essential for accurate species identification, and their advice is much appreciated. This study was possible because of the support of Professors David Warrell and David Theakston and a Wellcome Trust Travelling Fellowship.
None identified.
- 1. Sutherland SK, Leonard RL. Snakebite deaths in Australia 1992–1994 and a management update. Med J Aust 1995; 163: 616-618.
- 2. Currie BJ. Snakebite in tropical Australia, Papua New Guinea and Irian Jaya. Emerg Med (Fremantle) 2000; 12: 285-294.
- 3. Isbister GK, Currie BJ. Suspected snakebite: one year prospective study of emergency department presentations. Emerg Med (Fremantle) 2003; 15: 160-169.
- 4. Gow G. Graeme Gow’s complete guide to Australian snakes. Sydney: Angus and Robertson, 1989.
- 5. Cogger HG. Reptiles and amphibians of Australia. 6th ed. Sydney: Reed New Holland, 2000.
- 6. White J. Clinical toxicology of snakebite in Australia and New Guinea. In: Meier J, White J, eds. Handbook of clinical toxicology of animal venoms and poisons. Boca Raton: CRC Press, 1995: 595-617.
- 7. Epi-Info [computer program]. Version 6. Atlanta: Centers for Disease Control and Prevention, 1998.
- 8. Munro JG, Pearn JH. Snake bite in children: a five year population study from South-East Queensland. Aust Paediatr J 1978; 14: 248-253.
- 9. Jamieson R, Pearn J. An epidemiological and clinical study of snake-bites in childhood. Med J Aust 1989; 150: 698-702.
- 10. Lalloo DG, Trevett AJ, Saweri A, et al. The epidemiology of snakebite in Central Province and National Capital District, Papua New Guinea. Trans R Soc Trop Med Hyg 1995; 89: 178-182.
- 11. Warrell DA, Arnett C. The importance of bites by the Saw-scaled or Carpet Viper (Echis carinatus): epidemiological studies in Nigeria and a review of the world literature. Acta Tropica 1976; 33: 307-341.
- 12. Chippaux JP, Theakston RDG. Epidemiological studies of snake bite in French Guiana. Ann Trop Med Parasitol 1987; 81: 301-304.
- 13. Currie BJ, Sutherland SK, Hudson BJ, Smith AM. An epidemiological study of snake bite envenomation in Papua New Guinea. Med J Aust 1991; 154: 266-268.
- 14. Jelinek GA, Breheny FX. Ten years of snake bites at Fremantle Hospital. Med J Aust 1990; 153: 658-661.
- 15. Jelinek GA, Hamilton T, Hirsch RL. Admissions for suspected snake bite to the Perth adult teaching hospitals, 1979 to 1988. Med J Aust 1991; 155: 761-764.
- 16. Mead HJ, Jelinek GA. Suspected snakebite in children: a study of 156 patients over 10 years. Med J Aust 1996; 164: 467-470.
- 17. White J. Ophidian envenomation; a South Australian perspective. Rec Adelaide Child Hosp 1980-1981; 2: 311-421.
- 18. White J. A review of snakebites and suspected snakebites treated in South Australia with particular reference to reptile handlers. In: Gopalakrishnakone P, Tan CK, editors. Recent advances in toxinology. Singapore: Venom and Toxin Research Group, 1992.
- 19. Tibballs J. Diagnosis and treatment of confirmed and suspected snake bite. Implications from an analysis of 46 paediatric cases. Med J Aust 1992; 156: 270-274.
- 20. Fisher MM, Bowey CJ. Urban envenomation. Med J Aust 1989; 150: 695-698.
- 21. Barrett R, Little M. Five years of snake envenoming in far north Queensland. Emerg Med (Fremantle) 2003; 15: 500-510.
- 22. Flecker H. Snake bite in practice. Med J Aust 1940; 2: 8-13.
- 23. Sutherland SK, Tibballs J. Australian animal toxins. The creatures, their toxins and care of the poisoned patient. 2nd ed. Melbourne: OUP, 2001.
- 24. Campbell CH. The death adder (Acanthophis antarcticus): the effect of the bite and its treatment. Med J Aust 1966; 2: 922-925.
- 25. Lalloo DG, Trevett AJ, Black J, et al. Neurotoxicity, anticoagulant activity and evidence of rhabdomyolysis in patients bitten by death adders (Acanthophis sp.) in southern Papua New Guinea. Q J Med 1996; 89: 25-35.
- 26. Campbell CH. The taipan (Oxyuranus scutellatus) and the effect of its bite. Med J Aust 1967; 1: 735-739.
- 27. Lalloo DG, Trevett AJ, Korinhona A, et al. Snake bites by the Papuan taipan (Oxyuranus scutellatus canni); paralysis, hemostatic and electrocardiographic abnormalities, and effects of antivenom. Am J Trop Med Hyg 1995; 52: 525-531.
- 28. Wickramaratna JC, Fry BG, Aguilar MI, et al. Isolation and pharmacological characterization of a phospholipase A2 myotoxin from the venom of the Irian Jayan death adder (Acanthophis rugosus). Br J Pharmacol 2003; 138: 333-342.
- 29. Tibballs J, Sutherland SK, Rivera RA, Masci PP. The cardiovascular and haematological effects of purified prothrombin activator from the common brown snake (Pseudonaja textilis) and their antagonism with heparin. Anaesth Intensive Care 1992; 20: 28-32.






Abstract
Objective: To describe the epidemiology of snakebite in the “Top End” of the Northern Territory, and the envenoming syndromes of individual snake species.
Study design: Prospective collection of clinical data and snake identity.
Setting: Royal Darwin Hospital (RDH), a 300-bed tertiary hospital servicing a population of 140 000 spread over 522 561 km2.
Patients: All patients with bites by confirmed snake species between September 1989 and March 2003, and all suspected snakebite cases between September 1989 and March 1998.
Outcome measures: Incidence rates of definite snakebite and envenoming. Clinical features of bites from defined snake species.
Results: There were 348 suspected snakebites over 8.6 years, with 114 aerial evacuations to RDH, 216 patients (62%) definitely bitten (23.2/100 000 per year) and 79 (23%) envenomed (7.6/100 000 per year). There were 156 bites from confirmed species over 13.6 years: 31 (20%) from western brown snakes (Pseudonaja nuchalis), with early collapse in 14 (45%), consumptive coagulopathy in 26 (84%) and 25 (81%) given antivenom; 21 from death adders (Acanthophis spp.), with neurotoxicity in 8 (38%) and 6 (29%) given antivenom; and 20 from mulga snakes (Pseudechis australis), with local swelling in 19 (95%), myotoxicity in 12 (60%) and 15 (75%) given antivenom. In 34 bites from less venomous species, there was no life-threatening envenoming. There were no deaths.
Conclusions: Snakebite still causes morbidity in tropical Australia, but, with access to hospital and antivenom, deaths are rare. This study has enabled further definition of the envenoming syndromes of three highly venomous Australasian elapids.