Tiger snakes are one of the most frequent causes of envenoming in Australia, particularly in Victoria, and are the most important venomous snake in Tasmania. There are two species of tiger snake — Notechis scutatus (mainland tiger snake) and Notechis ater (black tiger snake) — although a recent taxonomic revision suggests the distinction may be somewhat arbitrary.1 Mainland tiger snakes average 1.2 metres in length and have significant variability in appearance, from the classical banded dark grey/black and yellow skin to unbanded types with a range of colours from black to light grey.2
Most previous reports on tiger snake bites have been case reports of one or a few cases,3-8 but there have been two case-series studies, one from South Australia9 and another from Western Australia.10 Both series were retrospective, with case confirmation based on clinical effects and on a positive result for tiger snake venom using the commercially available snake venom detection kit (sVDK).10 The frequency of different envenoming syndromes and the severity of clinical effects in tiger snake envenoming has not been fully characterised.
Tiger snake antivenom (TSAV) was the first snake antivenom to be released by the then Commonwealth Serum Laboratories (which later became CSL Ltd) in 1930. The initial dose was 1 vial. However, over the past few decades, the recommended dose has increased to 4 vials11 and, in some cases, many more are given.10,12 Recent laboratory and clinical studies have shown that far less antivenom is required to neutralise the effects of Australian snake venoms13,14 and to fully bind venom in vivo.15-17
We conducted a prospective cohort study of tiger snake envenomings recruited to the Australian Snakebite Project (ASP). The ASP is a prospective multicentre study of suspected snakebites or snake envenomings from over 100 Australian hospitals. The study design, recruitment and data collection have been described previously,15,18 and approval has been obtained from 19 human research and ethics committees covering all institutions involved.
Clinical envenoming syndromes were defined for each patient based on the clinical and laboratory features, using a previously developed classification: venom-induced consumption coagulopathy (VICC) (partial or complete), myotoxicity, neurotoxicity, thrombotic microangiopathy and systemic symptoms.19 In complete VICC, there is undetectable fibrinogen and/or a raised D-dimer concentration in citrate plasma (either at least 10 times the assay cut-off point or > 2.5 mg/L) and an international normalised ratio (INR) > 3.0. In partial VICC there is a low but detectable fibrinogen level, elevated D-dimer level and a maximum INR < 3.0. Myotoxicity is defined as an elevated creatine kinase (CK) level (> 1000 U/L) associated with myalgia and/or muscle tenderness.
The presence of any one of these syndromes was considered indicative of systemic envenoming. Clinical features of any hypersensitivity reactions were also documented and were graded according to Brown’s scale.20
Antivenom dose was defined as the amount given before the first post-antivenom blood collection for the measurement of venom. For patients given antivenom other than tiger snake antivenom, the following conversions were used (based on a previous study that showed that all terrestrial snake antivenoms produced by CSL Ltd are in fact polyvalent21): 1 vial of brown snake antivenom is equivalent to 0.6 vials of tiger snake antivenom, and 1 vial of polyvalent antivenom is equivalent to 4 vials of tiger snake antivenom. These are the average conversions, and there is variation from batch to batch.21
The method uses polyclonal antibodies (IgG) to each venom, raised in rabbits. Detection is by biotinylated antibodies followed by streptavidin horseradish peroxidase, as described elsewhere.17,22 The limit of detection for the assay is 0.15 ng/mL for individual venoms, and the assay is highly specific for each venom, except that Notechis and T. carinatus can only be distinguished from one another at concentrations above 2 ng/mL.16 This quantitative and specific EIA differs from the sVDK test, which is a qualitative EIA based on visual inspection of colour changes in wells. The latter is used to rapidly detect venom at the bite site or in urine.
The peak pre-antivenom tiger snake venom concentration was reported for each patient.
For descriptive statistics, median, range and interquartile range (IQR) were calculated for data not normally distributed. Proportions are given with 95% confidence intervals calculated using the Wilson’s procedure with a continuity correction.23 Statistical and graphical analyses were done using GraphPad Prism software, version 5.03 for Windows (GraphPad Software Inc, San Diego, Calif, USA).
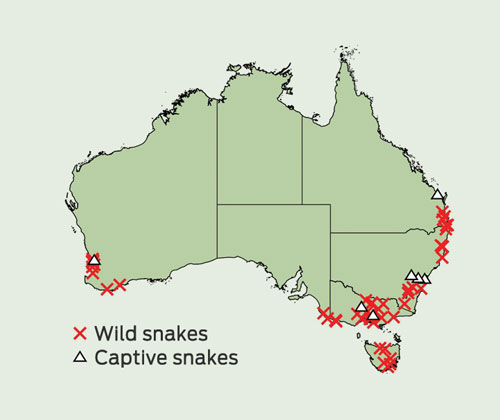
From 138 possible tiger snake bites and an additional four cases initially classified as brown snake bites, we identified 56 definite tiger snake envenoming cases and four definite non-envenomed tiger snake bites. The remaining 78 cases included 39 definite rough-scaled snakebites (based on EIA), 17 probable tiger snake bites (based on geography and sVDK tests without EIA confirmation), 18 cases in which there was either no venom EIA confirmation or the amount of venom detected by EIA was too low to distinguish between tiger snake or rough-scaled snake venom, one definite brown snake bite, and three inconclusive cases in which no venom was detected. Of the 56 definite tiger snake envenomings, the majority occurred in southern Australia and nine involved bites by captive snakes (Box 1).
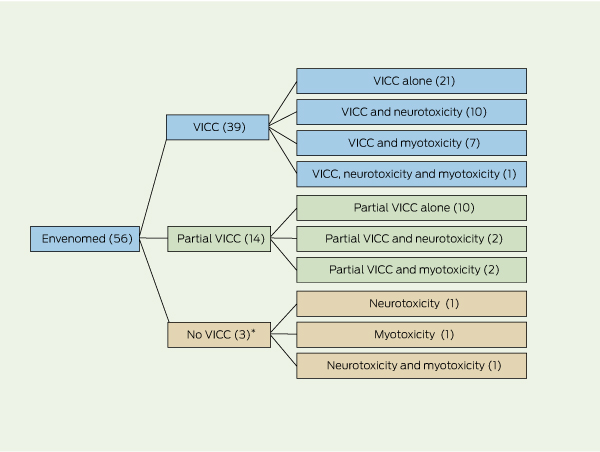
The patient demographic characteristics and clinical effects of the 56 tiger snake envenoming cases are summarised in Box 2 and Box 3. All envenomed patients developed VICC, neurotoxicity or myotoxicity, alone or in combination (Box 4). There was no relationship between the pattern of clinical syndromes and geographical location of the bite. There were no deaths. Five patients had an early collapse, including one associated with a seizure. One of the patients had a cardiac arrest, but also developed features of anaphylaxis before receiving antivenom and had a history of previous snakebites. Systemic symptoms occurred in 45 patients (80%), with nausea, vomiting and headache being most common (Box 3).
VICC was the commonest manifestation of envenoming. Thirty-nine patients had complete VICC. In these patients the median time until the INR returned to < 2.0 was 14.5 hours (IQR, 11.3–18.6 hours). Fourteen patients had partial VICC. Of the remaining three envenomed patients, one presented 8 days after the bite, one had a normal INR and a D-dimer level 10 times the upper limit of normal, and the third had a normal INR but fibrinogen and D-dimer levels were not tested. Bleeding occurred in 17 patients, and two of these had haematemesis without evidence of major gastrointestinal haemorrhage. There were no other major haemorrhages. Thrombotic microangiopathy, which occurred in three patients with VICC, was characterised by acute renal failure, thrombocytopenia and microangiopathic haemolytic anaemia (two of these cases have been reported in detail elsewhere24).
Neurotoxicity occurred in 17 patients (30%) and was severe in one patient, who had bulbar, respiratory and limb weakness requiring intubation and ventilation. The remaining 16 patients had mainly extraocular involvement (Box 3). Four of the 17 patients with neurotoxicity also had myotoxicity. Myotoxicity occurred in 11 patients and there was a raised CK level (> 1000 U/L) in another five. The median peak CK level was 4749 U/L (range, 1193–203 110 U/L). All 11 patients had local muscle pain and tenderness, six developed generalised myalgia or muscle tenderness, and one developed trismus. Acute renal failure did not develop in association with myotoxicity, except in one case where thrombotic microangiopathy was present. The patient with the most severe case of myotoxicity (who also developed severe neurotoxicity) had a peak CK level of 203 110 U/L but did not develop renal failure.
The clinical syndrome associated with tiger snake envenoming has been previously based on expert anecdotal reports and case reports. Although our results are generally consistent with current understanding of tiger snake envenoming, they provide an accurate description of tiger snake envenoming in a series of patients treated with early antivenom therapy. Tiger snake and rough-scaled snake envenoming syndromes were previously regarded as similar. Our results show that neurotoxicity is more common with tiger snake envenoming, occurring in almost a third of cases, compared with two out of 24 cases of rough-scaled snake envenoming reported in a previous study.16
Scop and colleagues reported on a 16-year retrospective series of 23 tiger snake envenoming cases.10 However, their inclusion criteria were based on a positive sVDK test, which we have shown here to be unreliable and thus unsuitable for the purpose of case definition in research studies. In addition, Scop and colleagues included only cases in which fibrinogen was undetectable, which biased the series towards patients with complete VICC.10 It is recognised that VICC can occur with only partial coagulation factor consumption,25 and in our study almost a third of patients with tiger snake envenoming had only partial VICC. An earlier series reported by White described 10 cases of tiger snake envenoming in which there was a similar range of effects.9
Our study brings into question the reliance on the sVDK test for determining appropriate antivenom treatment. The sVDK result was incorrect in 5/44 cases, giving a positive result for brown snake venom, which led to the incorrect use of antivenom in four of the five cases. The use of sVDK is thus problematic and may confuse clinical assessment. A “saving grace” in this situation is that, because all commercial antivenoms are polyvalent,21 brown snake antivenom contains sufficient TSAV to neutralise the procoagulant activity of tiger snake venom.26 However, this principle cannot be extended to the treatment of bites from other snakes that need much higher-volume antivenoms, such as the mulga snake, death adder and taipan. Although all commercial antivenoms are polyvalent, the low-volume monovalent antivenoms (for brown and tiger snake bites) may contain insufficient antivenom to treat bites from snakes that require higher-volume antivenoms. Knowledge about medically important snakes that occur in a region and the clinical syndromes they cause is required for the selection of appropriate antivenom.
The data presented here provide further evidence that 1 vial of antivenom is sufficient to treat envenoming, as initially recommended by CSL Ltd. Larger doses of antivenom have been recommended up until recently, based on in-vitro27 and clinical10 studies. An in-vitro study used venom concentrations that were 10 000-fold those seen in human snake envenoming. The study by Sprivulis and colleagues27 used a serum tiger snake venom concentration of 33 μg/mL, compared with the median serum concentration of 3.2 ng/mL found in our study. It is therefore not surprising that this study suggested that larger doses of antivenom were required. Previous clinical studies may have had unrealistic expectations about the time to recovery of clotting function and have reported the amount of antivenom given to patients, rather than determining the amount required to bind all antivenom in vivo, thus overestimating the required dose of antivenom. Our study provides in-vivo support for the claim that 1 vial of antivenom is an adequate dose. This is consistent with studies on other snakes15-17 and with in-vitro studies.14
1 Distribution of definite tiger snake envenoming cases, including bites by snakes in the wild and captive snakes
Received 8 October 2011, accepted 1 March 2012
- Geoffrey K Isbister
- Margaret A O’Leary
- Matthew Elliott
- Simon G A Brown
Tiger snakes are one of the most frequent causes of envenoming in Australia, particularly in Victoria, and are the most important venomous snake in Tasmania. There are two species of tiger snake — Notechis scutatus (mainland tiger snake) and Notechis ater (black tiger snake) ...
No relevant disclosures.
- 1. Rawlinson PA. Taxonomy and distribution of the Australian tiger snakes (Notechis) and copperheads (Austrelaps) (Serpentes: Elapidae). Proc R Soc Victoria 1991; 103: 125-135.
- 2. Cogger HG. Reptiles and amphibians of Australia. Sydney: Reed New Holland, 2000.
- 3. Tibballs J, Henning RD, Sutherland SK, Kerr AR. Fatal cerebral haemorrhage after tiger snake (Notechis scutatus) envenomation. Med J Aust 1991; 154: 275-276.
- 4. Sutherland SK, Coulter AR. Three instructive cases of tiger snake (Notechis scutatus) envenomation — and how a radioimmunoassay proved the diagnosis. Med J Aust 1977; 2: 177-180.
- 5. Penington A, Johnstone B. A case of local tissue necrosis following a bite by the Australian tiger snake Notechis scutatus. Aust N Z J Surg 1997; 67: 385-388.
- 6. Ferguson LA, Morling A, Moraes C, Baker R. Investigation of coagulopathy in three cases of tiger snake (Notechis ater occidentalis) envenomation. Pathology 2002; 34: 157-161.
- 7. Hood VL, Johnson JR. Acute renal failure with myoglobinuria after tiger snake bite. Med J Aust 1975; 2: 638-641.
- 8. Harvey PM, Tabrett DG, Solomons BJ, Thomas MA. Envenomation by a King Island tiger snake (Notechis ater humphreysi). Med J Aust 1982; 2: 192-193.
- 9. Scop J, Little M, Jelinek GA, Daly FF. Sixteen years of severe tiger snake (Notechis) envenoming in Perth, Western Australia. Anaesth Intensive Care 2009; 37: 613-618.
- 10. White J. A review of 105 cases of suspected snakebite in South Australia. In: Gopalakrishnakone P, Tan C, editors. Progress in venom and toxin research. Singapore: National University of Singapore, 1987: 15-19.
- 11. White J. CSL antivenom handbook. Melbourne: CSL, 2001.
- 12. Parkin JD, Ibrahim K, Dauer RJ, Braitberg G. Prothrombin activation in eastern tiger snake bite. Pathology 2002; 34: 162-166.
- 13. Isbister GK, Woods D, Alley S, et al. Endogenous thrombin potential as a novel method for the characterization of procoagulant snake venoms and the efficacy of antivenom. Toxicon 2010; 56: 75-85.
- 14. Isbister GK, O’Leary MA, Schneider JJ, et al. Efficacy of antivenom against the procoagulant effect of Australian brown snake (Pseudonaja sp.) venom: in vivo and in vitro studies. Toxicon 2007; 49: 57-67.
- 15. Churchman A, O’Leary MA, Buckley NA, et al. Clinical effects of red-bellied black snake (Pseudechis porphyriacus) envenoming and correlation with venom concentrations: Australian Snakebite Project (ASP-11). Med J Aust 2010; 193: 696-700. <MJA full text>
- 16. Gan M, O’Leary MA, Brown SG, et al. Envenoming by the rough-scaled snake (Tropidechis carinatus): a series of confirmed cases. Med J Aust 2009; 191: 183-186. <MJA full text>
- 17. Kulawickrama S, O’Leary MA, Hodgson WC, et al. Development of a sensitive enzyme immunoassay for measuring taipan venom in serum. Toxicon 2010; 55: 1510-1518.
- 18. Isbister GK, Brown SG, MacDonald E, et al; Australian Snakebite Project Investigators. Current use of Australian snake antivenoms and frequency of immediate-type hypersensitivity reactions and anaphylaxis. Med J Aust 2008; 188: 473-476. <MJA full text>
- 19. Isbister GK, White J, Currie BJ, et al; ASP Investigators. Clinical effects and treatment of envenoming by Hoplocephalus spp. snakes in Australia: Australian Snakebite Project (ASP-12). Toxicon 2011; 58: 634-640.
- 20. Brown SG. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol 2004; 114: 371-376.
- 21. O’Leary MA, Isbister GK. Commercial monovalent antivenoms in Australia are polyvalent. Toxicon 2009; 54: 192-195.
- 22. O’Leary MA, Isbister GK, Schneider JJ, et al. Enzyme immunoassays in brown snake (Pseudonaja spp.) envenoming: detecting venom, antivenom and venom–antivenom complexes. Toxicon 2006; 48: 4-11.
- 23. Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17: 857-872.
- 24. Casamento AJ, Isbister GK. Thrombotic microangiopathy in two tiger snake envenomations. Anaesth Intensive Care 2011; 39: 1124-1127.
- 25. Isbister GK, Scorgie FE, O’Leary MA, et al; ASP Investigators. Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10). J Thromb Haemost 2010; 8: 2504-2513.
- 26. O’Leary MA, Schneider JJ, Krishnan BP, et al. Cross-neutralisation of Australian brown and tiger snake venoms with commercial antivenoms: cross-reactivity or antivenom mixtures? Toxicon 2007; 50: 206-213.
- 27. Sprivulis P, Jelinek GA, Marshall L. Efficacy and potency of antivenoms in neutralizing the procoagulant effects of Australian snake venoms in dog and human plasma. Anaesth Intensive Care 1996; 24: 379-381.





Abstract
Objectives: To describe the clinical syndrome associated with definite tiger snake (Notechis spp) envenoming and to examine the ability of tiger snake antivenom (TSAV) to bind free venom in vivo.
Design, setting and participants: We conducted a prospective cohort study within the Australian Snakebite Project, reviewing all definite tiger snake envenoming cases between October 2004 and June 2011. Definite cases were identified by venom-specific enzyme immunoassay or expert snake identification.
Main outcome measures: Clinical effects of tiger snake envenoming; peak venom concentrations; number of vials of antivenom administered.
Results: Fifty-six definite tiger snake envenomings were identified. Clinical effects included venom-induced consumption coagulopathy (VICC) (n = 53), systemic symptoms (n = 45), myotoxicity (n = 11) and neurotoxicity (n = 17). Thrombotic microangiopathy occurred in three patients, all of whom developed acute renal failure. There were no deaths. A bite-site snake venom detection kit test was done in 44 patients, but was positive for tiger snake in only 33 cases. Fifty-three patients received TSAV and eight of these patients had immediate hypersensitivity reactions, severe enough in one case to satisfy diagnostic criteria for severe anaphylaxis. The median peak venom concentration in 50 patients with pretreatment blood samples available was 3.2 ng/mL (interquartile range [IQR], 1–12 ng/mL; range 0.17–152 ng/mL). In 49 patients with post-treatment blood samples available, no venom was detected in serum after the first antivenom dose. Ten patients were given 1 vial of TSAV; the median dose was 2 vials (range, 1–4 vials). Pretreatment serum venom concentrations did not vary significantly between patients given 1 vial of TSAV and those given 2 or more vials.
Conclusion: Tiger snake envenoming causes VICC, systemic symptoms, neurotoxicity and myotoxicity. One vial of TSAV, the dose originally recommended when the antivenom was first made available, appears to be sufficient to bind all circulating venom.