The lack of progress in improving health outcomes for Aboriginal and Torres Strait Islander (Indigenous) Australians has resulted in intensified efforts to close the life-expectancy gap within a generation.1 Despite having a 2.5-fold greater total disease burden than the non-Indigenous population,2 Indigenous Australians substantially underutilise primary health care consultations,3,4 pharmaceutical subsidies,3 and specialist care.5 Important barriers to improved primary health care might include this inadequate access to care, an insufficient capacity in health service systems,6 and dysfunctional patient–provider interactions.7
At least a third of the total Indigenous disease burden is attributable to vascular-related disease: cardiovascular disease (CVD), chronic kidney disease (CKD) and diabetes.2 Six risk factors (tobacco, high body mass index, high cholesterol level, physical inactivity, high blood pressure, and low fruit and vegetable intake) explain most of this vascular disease burden.2 Despite the availability of evidence-based therapies to manage these risk factors, significant evidence-practice gaps exist in both Indigenous8 and mainstream primary health care.9
Vascular disease prevention strategies based on a person’s overall or absolute cardiovascular risk maximise benefits and outperform the traditional approach which relies on management of single risk factors in isolation.10 Risk-based targeting of safe, effective drug therapies to those most likely to benefit could substantially reduce the total disease burden.11 There has been little research on the utility of absolute CVD risk-based management in Indigenous health care settings. Improved identification and management of high-risk individuals, based on adequate risk delineation, could provide a major opportunity to reduce the burden of disease in Indigenous people.
The Kanyini Vascular Collaboration is a national health services research program established to improve vascular disease outcomes for Indigenous people. The program’s first study, the Kanyini Audit, aimed to describe the identification and management of CVD risk in primary health care services and to identify opportunities for improvement.
An audit of a random sample of health care records of Indigenous Australian adults was conducted in collaboration with eight health services in New South Wales, Queensland, and Central Australia between October 2007 and May 2008. Sites with diverse service activity, based on size, location, funding and staffing, were selected.12 According to the Rural Remote Metropolitan Area classification (RRMA),13 two services were in capital cities (RRMA 1), two in major regional centres (RRMA 2–3), two in rural locations (RRMA 4–5), and two in remote communities (RRMA 7). Seven are Aboriginal Community Controlled Health Services and one is a state government Indigenous health service.
Based on the 1991 Anderson Framingham equation, we calculated 5-year CVD risk estimates for people aged ≥ 30 years (people aged < 30 years were excluded because the Anderson Framingham equation is not validated for this group).14 This equation uses age, sex, smoking status, blood pressure (BP), total and high-density lipoprotein (HDL) cholesterol levels, presence of diabetes, and presence of left ventricular hypertrophy to predict the risk of a first cardiovascular event (coronary heart disease, stroke, congestive heart failure and peripheral vascular disease). As the presence or absence of left ventricular hypertrophy is not reliably documented in primary care records, this was assumed to be absent.
We calculated risk using the most recent results available (including an average of the two most recent BP readings), whether or not the subjects were being treated for that risk factor. Recognising that the Framingham equation might underestimate risk for Indigenous populations,15 and acknowledging the absence of population-specific risk equations, the risk estimate was adjusted based on the 2004 National Heart Foundation of Australia (NHFA) recommendations.16 People with a recorded diagnosis of coronary heart disease, CVD, or peripheral vascular disease were assigned to the established CVD group. People with diabetes, an albumin : creatinine ratio > 3.0 mg/mmol, or an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73m2), as well as those aged ≥ 75 years, were automatically assigned a 5-year CVD risk ≥ 15%. For all others, a 5% upward adjustment to the Framingham risk estimate was applied.
The “screening gap” was defined as the proportion of people, recommended for screening for a particular risk factor, for whom no result of screening had been recorded at least once in the previous 2 years. Screening recommendations for our study were based on the National guide to a preventive health assessment in Aboriginal and Torres Strait Islander peoples17 and the Guidelines for preventive activities in general practice.18 Generally, these recommend screening Indigenous people for all the Framingham equation risk factors (except left ventricular hypertrophy) from the age of 18 years.17 Screening recommendations for proteinuria and CKD were based on Kidney Health Australia guidelines19 (with screening recommended for people with any one of the following: age > 50 years, systolic BP > 140 mmHg, diabetes, current smoker, body mass index ≥ 30 kg/m2).
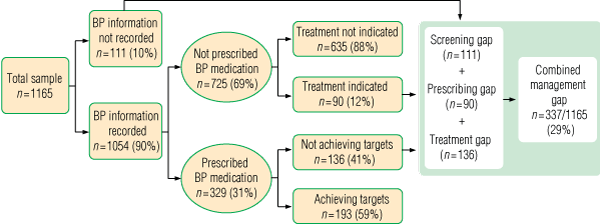
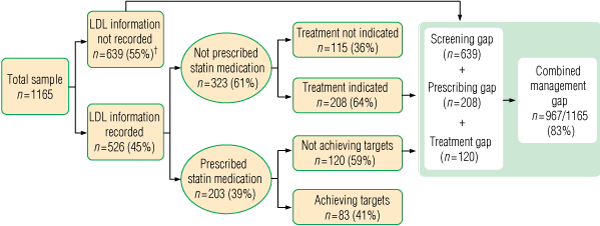
Two “prescribing gaps” were defined: (i) the proportion of people currently not prescribed BP medicines or statins, where the NHFA guidelines, current at the time of the audit,16,20 recommended treatment; and (ii) the proportion of people classified as being at high cardiovascular risk who were not prescribed BP medicines, statins or antiplatelet medicines. We also used Pharmaceutical Benefits Scheme (PBS) criteria for statin subsidies to assess prescribing gaps.21 The “treatment gap” was defined as the proportion of people, already prescribed BP medicines or statins, in whom target levels were not being reached. The study design did not allow for an assessment of adherence to medication regimens.
An overview of management practices and risk-factor screening gaps for each health centre is given in Box 1. Screening gaps occurred across all sites and were not related to the remoteness of services. Major contributors to incomplete absolute risk assessments were underscreening for cholesterol and albumin : creatinine ratio levels. Box 2 gives the risk-factor characteristics in people with relevant information available.
Prescribing of BP-lowering medicines and statin therapy, respectively, as measured against NHFA guidelines, are given in Box 3 and Box 4. For individuals currently not prescribed statins (Box 4) in whom NHFA guidelines recommend their being prescribed (n = 208), only 30% would qualify for subsidised treatment under PBS criteria.
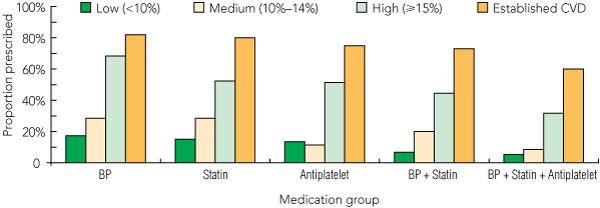
Box 5 examines prescribing gaps by CVD risk category. Forty per cent of people with established CVD were not prescribed combination therapy (BP medicines, statins, antiplatelets), and over half of the high-risk individuals without CVD were not prescribed both a BP medicine and a statin. When applying the 2004 NHFA guidelines for prescribing of BP- and lipid-lowering medications for individuals classified as high risk who have not yet experienced a cardiovascular event, treatment would not be recommended for 74% (95% CI, 64%–84%) and 30% (95% CI, 23%–39%), respectively, despite their high-risk status. Similarly, when applying PBS subsidy criteria for statin prescribing to this high-risk group who have not yet experienced a cardiovascular event, 30% would not qualify for the subsidy. Receipt of a Medicare preventive health check was not significantly associated with improved prescribing of BP medicines or statins for high-risk individuals.
The first of these was the large screening gaps. Although the absolute risk-based approach has theoretical benefits, its utility was limited by under-ascertainment of risk. The screening gaps were broadly similar to those found in mainstream general practice settings,22 and were especially large for younger, less frequent attendees. They were not related to remote location. It was encouraging that Medicare Health Assessments were associated with significantly smaller screening gaps. Point-of-care testing could be an effective strategy to ameliorate the under-performing of cholesterol and albumin : creatinine ratio tests. Although a national point-of-care program exists,23 cholesterol testing does not attract a Medicare rebate for health services participating in this program.
Substantial prescribing gaps were encountered for those at highest risk of a CVD event, although risk management compares favourably with previous Australian and New Zealand studies.9,22,24,25 Importantly, around a third of high-risk people without CVD were classified by both NHFA guidelines and PBS subsidy criteria as not qualifying for statin therapy, and almost three-quarters did not qualify for prescribing of BP medications under the 2004 NHFA guidelines; this could be considered a “guideline gap”. Although the changes in the 2008 NHFA guidelines will assist BP management, substantial gaps remain in recommendations for lipid-lowering drugs. This supports the findings of Chen et al that there is not only a need for improvement in guideline adherence, but for guidelines themselves to adequately identify appropriate individuals for treatment.26
The barriers highlighted above are not restricted to Indigenous health care. Although the large variability in Aboriginal and Torres Strait Islander health services may mean these findings are not representative of all service settings, the similarity in findings with mainstream general practice22 suggests these barriers are prevalent across the primary health care system.
Based on our study’s findings, we endorse current recommendations that an absolute risk-based approach to screening be recommended for Indigenous adults, and that this be incorporated into a preventive health assessment.17 This check should include the Framingham risk factors (with optional assessment of left ventricular hypertrophy) — albumin : creatinine ratio, eGFR, body mass index and waist circumference, and blood glucose level for people without diabetes, and glycated haemoglobin (HbA1c) level for those with diabetes. Although it might be reasonable to assume that younger people are less likely to have a CVD event (which might partially explain the large screening gaps in younger people), the sharp rise in Indigenous CVD mortality after the age of 30 provides a strong argument for comprehensive screening from at least this age. Although a national absolute risk-based screening guideline has recently been released,27 a single risk-management guideline now needs to be developed to address prescribing gaps. It must avoid the assumption that all Indigenous people are at high risk, and be linked to appropriate resourcing to support its implementation. Given the evolving and uncertain evidence base, regular revision of the guideline would be essential.
1 Care practices and screening gaps at audited health centres, by health centre location (two centres at each location)
2 Vascular risk-factor characteristics of patients obtained from health records
Family history of coronary heart disease in a first-degree relative† |
|||||||||||||||
3 Blood pressure (BP) management measured against 2004 National Heart Foundation of Australia guidelines*16
 | |||||||||||||||
|
Except where indicated, the denominator for each percentage is the number in the preceding step. | |||||||||||||||
Received 8 February 2009, accepted 2 June 2009
- David P Peiris1
- Anushka A Patel1
- Alan Cass1
- Michael P Howard2
- Maria L Tchan1
- John P Brady3
- Joanne De Vries4
- Bernadette A Rickards2
- Della J Yarnold1
- Noel E Hayman10
- Alex D Brown2
- 1 The George Institute for International Health, University of Sydney, Sydney, NSW.
- 2 Centre for Indigenous Vascular and Diabetes Research, Baker IDI Heart and Diabetes Institute, Alice Springs, NT.
- 3 Inala Indigenous Health Service, Queensland Health, Brisbane, QLD.
- 4 Wuchopperen Health Service, Manoora, QLD.
On behalf of the Kanyini Vascular Collaboration, the writing committee thanks all health service collaborators and their governing bodies for participating in this work. The Kanyini Vascular Collaboration is funded by a National Health and Medical Research Council (NHMRC) health services research grant (Grant ID #402797). The Collaboration chief investigators, associate investigators and health service representatives include (in alphabetical order): John Boffa, Alex Brown, Alan Cass, Jeannie Devitt, Sandra Eades, Noel Hayman, Nicole Isbel, Stephen Jan, Nancy Long, Peter O’Mara, Anushka Patel, Cilla Preece, Ian Ring, Karmananda Saraswati, Janelle Speed, Greg Stewart, Susan Thomas, Andrew Tonkin, Vicki Wade, Tarun Weeramanthri, Darryl Wright. Full details of the program are available at
Anushka Patel has received lecture fees and travel assistance from companies manufacturing and marketing blood pressure lowering drugs and/or statins, including Servier, Pfizer, Abbott, AstraZeneca and Merck Sharp & Dohme. Alex Brown received travel support from Alphapharm to present preliminary findings of this project at the Cardiac Society of Australia and New Zealand 2008 conference.
- 1. Aboriginal and Torres Strait Islander Social Justice Commissioner and the Steering Committee for Indigenous Health Equality. Close the Gap. National Indigenous health equality targets. Outcomes from the National Indigenous Health Equality Summit. http://www.hreoc.gov.au/social_justice/health/targets/health_targets.pdf (accessed Aug 2009).
- 2. Vos T, Barker B, Stanley L, Lopez AD. The burden of disease and injury in Aboriginal and Torres Strait Islander peoples, 2003. Brisbane: University of Queensland, 2007.
- 3. Australian Institute of Health and Welfare. Expenditures on health for Aboriginal and Torres Strait Islander peoples, 2004–05. Health and welfare expenditure series no. 33. Canberra: AIHW, 2008. (AIHW Cat. No. HWE 40.)
- 4. Kelaher M, Dunt D, Thomas D, Anderson I. Comparison of the uptake of health assessment items for Aboriginal and Torres Strait Islander people and other Australians: implications for policy. Aust New Zealand Health Policy 2005; 2: 21. doi: 10.1186/1743-8462-2-21.
- 5. Cunningham J. Diagnostic and therapeutic procedures among Australian hospital patients identified as Indigenous. Med J Aust 2002; 176: 58-62. <MJA full text>
- 6. Si D, Bailie R, Cunningham J, et al. Describing and analysing primary health care system support for chronic illness care in Indigenous communities in Australia’s Northern Territory — use of the Chronic Care Model. BMC Health Serv Res 2008; 8: 112.
- 7. Cass A, Lowell A, Christie M, et al. Sharing the true stories: improving communication between Aboriginal patients and healthcare workers. Med J Aust 2002; 176: 466-470. <MJA full text>
- 8. Si D, Bailie R, Connors C, et al. Assessing health care systems for guiding improvement in diabetes care. BMC Health Serv Res 2005; 5: 56. doi: 10.1186/1472-6963-5-56.
- 9. Vale MJ, Jelinek MV, Best JD. How many patients with coronary heart disease are not achieving their risk-factor targets? Experience in Victoria 1996–1998 versus 1999–2000. Med J Aust 2002; 176: 211-215. <MJA full text>
- 10. Jackson R, Lawes C, Bennet D, et al. Treatment with drugs to lower blood pressure and blood cholesterol based on an individual’s absolute cardiovascular risk. Lancet 2005; 365: 434-441.
- 11. Jackson R, Wells S, Rodgers A. Will screening individuals at high risk of cardiovascular events deliver large benefits? Yes. BMJ 2008; 337: a1371. doi: 10.1136/bmj.a1371.
- 12. Australian Government Department of Health and Ageing, National Aboriginal Community Controlled Health Organisation. A national profile of Australian Government funded Aboriginal and Torres Strait Islander primary health care services. Service activity reporting. 2005–06 key results. Canberra: DoHA, 2008.
- 13. Australian Institute of Health and Welfare. Rural, regional and remote health: a guide to remoteness classifications. Rural health series no. 4. Canberra: AIHW, 2004. (AIHW Cat. No. PHE 53.) http://www.aihw.gov.au/publications/index.cfm/title/9993 (accessed Jun 2009).
- 14. Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease profiles. Am Heart J 1991; 121: 293-298.
- 15. Wang Z, Hoy WE. Is the Framingham coronary heart disease absolute risk function applicable to Aboriginal people? Med J Aust 2005; 182: 66-69. <MJA full text>
- 16. National Heart Foundation of Australia. Hypertension management for doctors 2004. Canberra: NHFA, 2003. http://www.heartfoundtion.org.au/SiteCollectionDocuments/hypertension%20management%20guide.pdf (accessed Aug 2009).
- 17. National Aboriginal Community Controlled Health Organisation. National guide to a preventive health assessment in Aboriginal and Torres Strait Islander peoples. Melbourne: Royal Australian College of General Practitioners, 2005.
- 18. Royal Australian College of General Practitioners. Guidelines for preventive activities in general practice. 6th ed. Melbourne: RACGP, 2005.
- 19. Kidney Health Australia. Chronic kidney disease (CKD) management in general practice. Melbourne: KHA, 2007.
- 20. National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand. Position statement on lipid management, 2005. Heart Lung Circ 2005; 14: 275-291.
- 21. Australian Government Department of Health and Ageing. PBS-eligibility criteria for lipid lowering drugs fact sheet. Canberra: DoHA, 2006. http://www.health.gov.au/internet/main/publishing.nsf/Content/lipid_eligibilitycriteria.htm (accessed Apr 2009).
- 22. Webster RJ, Heeley EL, Peiris DP, et al. Gaps in cardiovascular disease risk management in Australian general practice. Med J Aust 2009; 191: 324-329.
- 23. Martin DD, Shephard MDS, Freeman H, et al. Point-of-care testing of HbA1c and blood glucose in a remote Aboriginal Australian community. Med J Aust 2005; 182: 524-527. <MJA full text>
- 24. Peiris D, Murray J, Scully D, et al. Cardiovascular risk management at a Maori-led primary health organisation — findings from a cross-sectional audit. N Z Med J 2008; 121: 35-46.
- 25. Reid C, Nelson MR, Shiel L, et al. Australians at risk: management of cardiovascular risk factors in the REACH Registry. Heart Lung Circ 2008; 17: 114-118.
- 26. Chen L, Rogers SL, Colagiuri S, et al. How do the Australian guidelines for lipid-lowering drugs perform in practice? Cardiovascular disease risk in the AusDiab Study, 1999–2000. Med J Aust 2008; 189: 319-322. <MJA full text>
- 27. National Heart Foundation of Australia. National Vascular Disease Prevention Alliance. Guidelines for the assessment of absolute cardiovascular disease risk. Canberra: NHFA, 2009. http://www.heartfoundation.org.au/SiteCollectionDocuments/A_AR_Guidelines_FINAL %20FOR%20WEB.pdf (accessed Jun 2009).





Abstract
Objective: To describe cardiovascular disease (CVD) risk management in Indigenous primary health care.
Design, setting and participants: Review of 1165 randomly selected case records of Indigenous Australian adults, aged ≥ 18 years, regularly attending eight health services in diverse settings in New South Wales, Queensland and Central Australia, October 2007 – May 2008.
Main outcome measure: Adherence to CVD risk screening and management guidelines, especially with respect to overall or absolute CVD risk.
Results: More than half the people in the sample (53%) were not adequately screened for CVD risk according to national recommendations. Underscreening was significantly associated with younger age, less frequent attendance, and lower uptake of the Medicare Health Assessment. Of the sample, 9% had established CVD, and 29% of those aged ≥ 30 years were classified as high risk according to the 2004 National Heart Foundation of Australia (NHFA) adjusted Framingham equation. Of those with CVD, 40% (95% CI, 30%–50%) were not prescribed a combination of blood pressure (BP) medicines, statins and antiplatelet agents, and 56% (95% CI, 49%–62%) of high-risk individuals without CVD were not prescribed BP medicines and statins. For high-risk individuals not prescribed BP medicines or statins, 74% (95% CI, 64%–84%) and 30% (95% CI, 23%–39%) respectively, did not meet 2004 NHFA criteria for prescribing of these medications, and of those already prescribed BP medicines or statins, 41% (95% CI, 36%–47%) and 59% (95% CI, 52%–66%) did not meet respective guideline targets.
Conclusions: These management gaps are similar to those found in non-Indigenous health care settings, suggesting deficiencies across the health system. Prescribing guidelines which exclude many high-risk individuals contribute to suboptimal management. Guideline reform and improved health service capacity could substantially improve Indigenous vascular health.