Type 2 diabetes mellitus and its sequelae are a major cause of premature mortality in Aboriginal Australians and Torres Strait Islanders today.1 Whereas this disease did not seem to exist in Australia before European settlement,2 reported prevalence rates in the last decade have ranged between 10% and 30%, depending on the study populations and screening methods, and have shown an increasing trend.3
Effective diagnostic and management tools are needed. From the viewpoints of the community members and their on-site carers, an ideal diabetes monitoring program would combine immediate and easily interpretable results with direct feedback to the individual, and would be linked to an effective long-term follow-up program. Point-of-care (POC) testing of blood glucose and glycosylated haemoglobin (HbA1c) levels would meet these requirements if shown to be accurate and reliable in the remote, hot and humid conditions characteristic of many Indigenous communities.
The Bayer DCA 2000+ glycohaemoglobin analyser (Bayer Australia, Melbourne, Vic) is being increasingly used for POC HbA1c testing in remote and rural clinical settings, through the Australian Government’s Quality Assurance for Aboriginal Medical Services (QAAMS) Program,4,5 which now involves over 60 services across Australia. The HemoCue Glucose 201 analyser (Medipac Scientific, Sydney, NSW) is a new-generation, hand-held glucose meter that is now widely used in Australia.
We conducted a study in a remote Indigenous community in northern Western Australia to examine the accuracy of POC capillary HbA1c and glucose measurements for monitoring diabetes in difficult environmental working conditions, with extreme heat and humidity.
This project was part of a community-based capacity-building program designed by the Unity of First Peoples of Australia (UFPA) and Western Australian Country Health Services — Kimberley region, to improve primary and secondary prevention of chronic metabolic diseases in Indigenous Australian communities.
For the 2 months preceding the study week, three experienced UFPA carers (D D M, H F and G P M) lived in the community to establish a good relationship with community members, gather population statistics, assess and optimise knowledge about diabetes and lifestyle, and prepare the community for the assessment.
Participants were interviewed to obtain a basic medical history and underwent a physical examination. They were asked to fast overnight (unless currently receiving medication for diabetes) before collection of blood and urine samples the following morning for POC and laboratory investigations.
All participants had POC measurement of fasting capillary glucose level. Those with a glucose level < 5.0 mmol/L (equivalent to fasting venous plasma glucose level < 5.5 mmol/L6) were assumed not to have diabetes and not tested further (unless known to be taking medication for diabetes).
Participants with a fasting capillary glucose level ≥ 5.0 mmol/L, and those with self-reported diabetes, were immediately followed up with POC capillary HbA1c assay of the same capillary blood sample and with venepuncture for subsequent measurement of HbA1c and glucose levels in a reference laboratory.
Participants with a laboratory venous plasma glucose level in the range 5.5–11.1 mmol/L underwent an oral glucose tolerance test (OGTT) with 75 g of diluted anhydrous glucose on a subsequent day.
Diabetes was defined as:
Capillary glucose level was measured on site in a 5 μL fingerprick blood sample by a HemoCue Glucose 201 analyser. This measures glucose enzymatically using glucose dehydrogenase and produces a result within 4 minutes.
Capillary HbA1c was measured on site in a 1 μL sample of whole blood by a Bayer DCA 2000+ analyser. This measures HbA1c immunochemically, producing a result in 6 minutes.9 Blood samples for HbA1c testing were transferred to reagent cartridges and analysed immediately after collection to ensure they did not dry out, causing measurement errors.
POC analyses were performed in a room open to the outside environment, in which temperature varied between 27°C and 31°C. For HbA1c measurement, which can be affected by high temperature, we followed the manufacturer’s recommendations to check that reagents had not been exposed to excessive heat (indicated by a heat-sensitive colour pad on the front of each reagent box), and to recalibrate the analyser and test a quality control sample each time a new box of reagents was opened.
Laboratory tests were performed at Derby PathCentre, Derby, WA (glucose), and the Western Australian Centre for Pathology and Medical Research, Perth, WA (HbA1c).
For glucose analysis, venous whole blood samples were collected in containers with fluoride-EDTA as preservative, then centrifuged at room temperature for 10 minutes at ≥ 800 g. Supernatants were stored at 0°C for less than 4 hours before being transported on ice by road to Derby (3–4 hours’ drive). Venous plasma glucose level was measured enzymatically on the Vitros 250 Analyser (OrthoClinical Diagnostics, Rochester, NY, USA) using glucose oxidase spectrophotometric dry chemistry.
For HbA1c measurement, part of each original whole blood sample was transferred to a container with EDTA as preservative, and flown on ice 2000 km to Perth. HbA1c was measured using cation-exchange high performance liquid chromatography (HPLC) on the Bio-Rad Variant II (Bio-Rad Laboratories, Hercules, USA). This has mean intra- and inter-assay precision (coefficients of variation) < 2%. This assay is certified by the US National Glycohemoglobin Standardization Program as traceable to the Diabetes Control and Complications Trial reference method.10
Laboratory results were available after 1 day for glucose, and after 3 days for HbA1c.
Data were analysed using JMP software11 and are presented as mean and 95% confidence intervals unless otherwise stated. Linear regression analysis was performed and Pearson’s correlation coefficient (r) was calculated for each analyte. The two-tailed Student’s t test was then used to compare POC and laboratory measurements for paired samples, with P < 0.05 representing statistical significance. Bland and Altman plots12 were used to calculate mean difference (bias) and limits of agreement (LOA) between the two methods. Regression analysis was performed on the Bland and Altman plots to determine whether bias was constant or proportional to concentration.
Power calculations using 0.05 for α and β suggested that 26 participants would be required to detect a 1 mmol/L difference in means between laboratory plasma glucose and POC capillary glucose levels, and that 12 participants would be required to detect a 0.5% difference between laboratory and POC HbA1c results.
POC capillary glucose measurements were made in 152 individuals, including 40 children aged between 11 and 18 years (Box 1). These 152 represented 76% of the population aged over 11 years in the community, and included 82% of residents with known diabetes. POC capillary HbA1c measurements and laboratory analyses of venous blood were subsequently performed in 88 of these people (all with capillary glucose level ≥ 5.0 mmol/L or self-reported diabetes).
Prevalence of diabetes was found to be 32% in adults (36 of 112) and 0 in children. The prevalence of impaired fasting glucose could not be reliably assessed as 40% of participants were not properly fasting. Similarly, the prevalence of impaired glucose tolerance was not calculated as the OGTT was performed only in participants with fasting plasma glucose levels in the range 5.5–11 mmol/L. None of the patients with diabetes were receiving renal dialysis treatment at the time of the study.
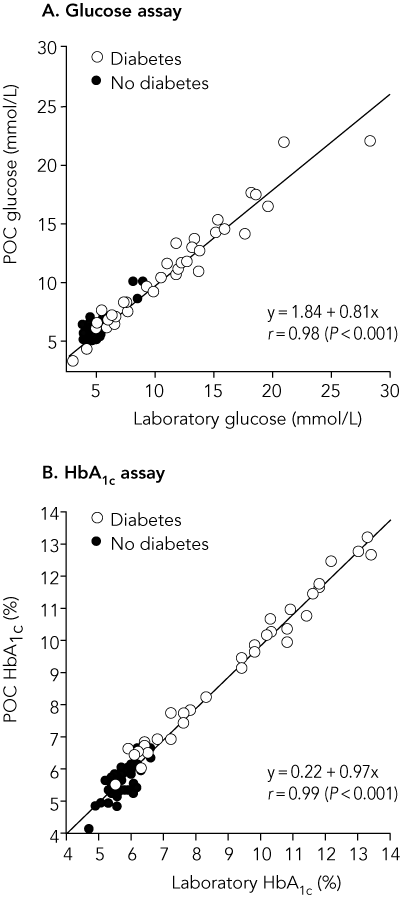
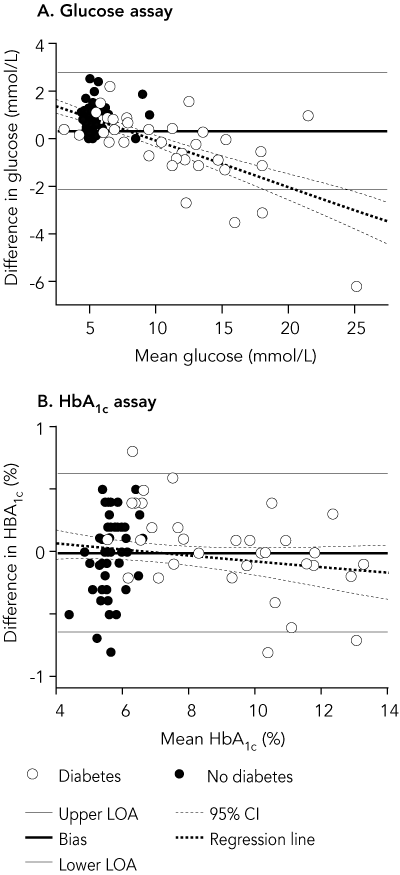
POC capillary glucose level is compared with laboratory plasma glucose level for the same patients in Box 2. Glucose concentrations determined by the two methods were significantly correlated (r = 0.98; P < 0.001) (Box 3A). However, actual values differed significantly between the two methods (P = 0.007 by paired t test). The mean difference was + 0.36 mmol/L (95% CI, 0.13–0.62 mmol/L), with lower and upper LOA, − 2.07 and 2.79 mmol/L (Box 4A). The difference in glucose concentration between the two methods was concentration dependent (r = 0.69; P < 0.001), with the POC measurement generally higher than the laboratory measurement at glucose concentrations < 10 mmol/L by POC measurement, and lower at concentrations > 10 mmol/L.
POC capillary HbA1c concentration is compared with laboratory plasma HbA1c concentration in the same patients in Box 2. Median values for HbA1c concentration by the two methods were identical (6.0%), as were mean values (7.1%). Results by the two methods were significantly correlated (r = 0.99; P < 0.001) (Box 3B), and the mean difference between them was neither statistically nor clinically significant (0.002%; 95% CI, − 0.07% to 0.07%; LOA, − 0.66% to 0.66%; P = 0.95 by paired t test) (Box 4B). The difference was greater than 0.5% in five of the 88 samples, only one of which was in the HbA1c range 6%–10%. The very small bias observed was constant across the range of HbA1c concentrations measured (r = 0.05; P = 0.14).
Indigenous Australians in regional Australia often live in isolated communities that are a significant distance from pathology laboratories. For example, in our study, the nearest laboratories able to measure glucose and HbA1c concentrations were 300 km and 2000 km away, respectively. POC pathology testing is therefore a desirable alternative to laboratory testing, provided it gives comparable results. Our study aimed to assess the accuracy and reliability of POC glucose and HbA1c tests compared with laboratory tests of venous samples transported to the nearest laboratory.
For HbA1c, the values obtained by POC and laboratory testing were statistically, analytically and clinically identical. Thus, POC testing for HbA1c using the Bayer DCA 2000+ analyser has demonstrated acceptable accuracy for field use in this remote Australian Aboriginal community. However, we could not assess the precision (or reproducibility) of these tests, because of the small number of quality control samples tested. Certainly, it is important that the precision of HbA1c measurement approaches 3% or less, to ensure that clinically significant changes in serial HbA1c concentrations can be detected.13 In the QAAMS Program (currently being conducted in 60 Aboriginal medical services across Australia), precision of HbA1c measurement using the Bayer DCA 2000+ is monitored continually. For the past 5 years, the median between-site precision has averaged 3.5%,4 while during 2004 it averaged 2.9%.14
We found that the POC and laboratory results for glucose concentration were reasonably correlated but showed a concentration-dependent difference. Many variables could account for this. The time available for training local staff to use the HemoCue glucose meter was very limited; appropriate and detailed training is critical for staff conducting tests outside the laboratory environment. Other factors potentially contributing to the observed difference include the different samples collected (capillary versus venous blood) and tested (whole blood versus centrifuged plasma), and the effects of transportation on the laboratory samples.6 A further study comparing glucose concentrations measured by the HemoCue meter and the Vitros Analyser using the same venous samples after appropriate staff training would be necessary to fully investigate the observed differences.
We did not formally survey the satisfaction of patients and health professionals with POC testing. However, non-laboratory staff in the community (Aboriginal health workers and nurses) were able to operate the DCA 2000+ after on-site training. POC testing provides the opportunity for immediate feedback and counselling, making it an ideal tool for inexpensive on-site motivational management of diabetes. Patients expressed their appreciation of the simultaneous education and opportunity to “see what happens with their blood”. They generally preferred fingerprick collection to venepuncture. Other studies in both Indigenous and non-Indigenous settings have also shown that POC HbA1c testing can improve diabetes control when linked with aggressive clinical management regimens and specialist support.15-17
This study shows that HbA1c can be conveniently and accurately measured by POC testing with the Bayer DCA 2000+ analyser in rural and remote clinical settings. This form of testing is suitable for regular HbA1c monitoring across all concentrations. The study also opens the way to investigate the contribution of POC HbA1c testing to diagnosis of diabetes when it is uncertain whether the patient has fasted. POC glucose testing has a useful role in screening for diabetes risk, as well as self-monitoring for people with known diabetes, and it is important that the performance of different glucose meters is known in specific clinical settings. However, the diagnosis of diabetes should still rely on confirmatory tests of plasma glucose concentration in the laboratory.6
1 Characteristics of study participants (n = 152), and point-of-care (POC) and laboratory results for those who had both POC and laboratory testing (n = 88)*
|
|
Adults |
Children (non-diabetic) |
||||||||||||
Variable |
|
Diabetic |
Non-diabetic |
||||||||||||
Total |
|
(n = 36) |
(n = 76) |
(n = 40) |
|||||||||||
No. of females (%) |
|
27 (75%) |
42 (53%) |
23 (57%) |
|||||||||||
Age in years |
Mean (SD) |
50.5 (14.1) |
37.5 (15.4) |
13.7 (1.7) |
|||||||||||
|
Range |
(18.4–76.3) |
(27.3–76.3) |
(11.0–17.5) |
|||||||||||
Diabetes or POC glucose ≥ 5.0 mmol/L* |
(n = 36) |
(n = 38) |
(n = 14) |
||||||||||||
POC glucose (mmol/L) |
Mean (SD) |
11.16 (4.58) |
5.87 (1.04) |
5.87 (1.30) |
|||||||||||
|
Median (range) |
11.0 (3.4–22.1) |
5.5 (4.5–10.1) |
5.3 (5.1–10.1) |
|||||||||||
Laboratory glucose (mmol/L) |
Mean (SD) |
11.50 (5.62) |
5.23 (1.00) |
4.71 (1.03) |
|||||||||||
Median (range) |
11.8 (3.0–28.3) |
5.0 (3.9–9.1) |
4.5 (4.0–8.2) |
||||||||||||
POC HbA1c (%) |
Mean (SD) |
9.17 (2.22) |
5.80 (0.41) |
5.33 (0.42) |
|||||||||||
|
Median (range) |
9.5 (5.6–13.2) |
5.8 (4.9–6.7) |
5.4 (4.2–5.8) |
|||||||||||
Laboratory HbA1c (%) |
Mean (SD) |
9.15 (2.40) |
5.74 (0.38) |
5.53 (0.34) |
|||||||||||
|
Median (range) |
9.4 (5.5–13.4) |
5.7 (4.9–6.6) |
5.6 (4.7–6.1) |
|||||||||||
* POC assay of HbA1c and laboratory assay of both glucose and HbA1c were conducted only for people with self-reported diabetes or POC glucose level ≥ 5.0 mmol/L. HbA1c = glycosylated haemoglobin. |
|||||||||||||||
2 Comparison of point-of-care and laboratory results for 88 participants with capillary glucose level ≥ 5.0 mmol/L or known diabetes
|
Glucose (mmol/L) |
HbA1c (%) |
|||||||||||||
|
POC |
Laboratory |
POC |
Laboratory |
|||||||||||
Mean |
7.99 |
7.63 |
7.06 |
7.06 |
|||||||||||
Median |
6.25 |
5.35 |
6.0 |
6.0 |
|||||||||||
Range |
3.4–22.2 |
3.0–28.3 |
4.2–13.2 |
4.7–13.4 |
|||||||||||
Mean difference (95% CI) |
+ 0.36 (0.13–0.62) (P = 0.007)* |
0.002 (− 0.07 to 0.07) (P = 0.95)* |
|||||||||||||
Limits of agreement |
− 2.07 to 2.79 |
− 0.66 to 0.66 |
|||||||||||||
* By paired t test. POC = point of care. HbA1c = glycosylated haemoglobin. |
|||||||||||||||
Received 19 October 2004, accepted 31 March 2005
- David D Martin1
- Timothy W Jones2
- Elizabeth A Davis3
- Mark D S Shephard4
- Hayley Freeman5
- Graeme P Maguire6
- Max K Bulsara7
- 1 Endocrinology and Diabetes, Princess Margaret Hospital for Children, Perth, WA.
- 2 Community Point-of-Care Services, Flinders University Rural Clinical School, Adelaide, SA.
- 3 Western Australian Country Health Services — Kimberley Region, Broome, WA.
- 4 School of Population Health and Telethon Institute of Child Health Research, The University of Western Australia, Subiaco, WA.
We thank the members of the community involved in the study, and the Hon Mr Ernie Bridge and his team at the Unity of First Peoples of Australia (UFPA) for their commitment and expertise in the initiation, organisation and coordination of the UFPA diabetes program. We thank Dean Whiting (Manager, Near Patient Testing, Bayer Australia) for the loan of a DCA 2000+ analyser, and for reagents and control specimens, and Michelle Arnebark (Managing Director, Medipac Scientific) for providing HemoCue glucose meters.
David Martin thanks Novo Nordisk for a clinical and research fellowship at Princess Margaret Hospital for Children. We thank Servier Laboratories (Australia) and Bayer Australia for their sponsorship and support of the Community Point-of-Care Services Unit at the Flinders University Rural Clinical School.
The supporting sources (see Acknowledgements) had no role in study design, data collection, analysis or interpretation, or in writing the article.
- 1. The health and welfare of Australia’s Aboriginal and Torres Strait Islander peoples 2003. Canberra: Australian Bureau of Statistics, Australian Institute of Health and Welfare, 2003.
- 2. De Couten M, Hodge A, Docose G, et al. Review of the epidemiology, aetiology, pathogenesis and preventability of diabetes in Aboriginal and Torres Strait Islander populations. Canberra: Office for Aboriginal and Torres Strait Islander Health Services, Commonwealth Department of Health and Family Services, 1998.
- 3. Daniel M, Rowley KG, McDermott R, et al. Diabetes incidence in an Australian Aboriginal population. An 8-year follow-up study. Diabetes Care 1999; 22: 1993-1998.
- 4. Shephard M, Gill J. Results of an innovative education, training and quality assurance program for point-of-care HbA1c testing using the Bayer DCA 2000 in Australian Aboriginal community controlled health services. Clin Biochem Rev 2003; 24: 123-131.
- 5. Shephard M, Mundraby K. Assisting diabetes management through point-of-care HbA1c testing - the ‘QAAMS’ program for Aboriginal health workers. Aborig Isl Health Work J 2003; 27: 12-18.
- 6. National Health and Medical Research Council. National evidence based guidelines for the management of type 2 diabetes mellitus. Primary prevention, case detection and diagnosis, 2001. Available at: http://www.health.gov.au/nhmrc/publications/pdf/cp86.pdf (accessed Apr 2005).
- 7. World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Geneva: WHO Department of Noncommunicable Disease Surveillance, 1999.
- 8. The expert committee on the diagnosis and classification of diabetes mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26: 3160-3167.
- 9. John WG, Edwards R, Price CP. Laboratory evaluation of the DCA 2000 clinic HbA1c immunoassay analyser. Ann Clin Biochem 1994; 31: 367-370.
- 10. Little RR, Rohlfing CL, Wiedmeyer HM, et al. The national glycohemoglobin standardization program: a five-year progress report. Clin Chem 2001; 47: 1985-1992.
- 11. SAS Institute. JMP software release 5.01. Cary, NC: SAS Institute.
- 12. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307-310.
- 13. White GH, Farrance I. Uncertainty of measurement in quantitative medical testing. Clin Biochem Rev 2004; 25 Suppl 2: S1-S24.
- 14. Shephard M. Quality assurance program for DCA 2000 HbA1c testing in Aboriginal medical services. Fourth progress report, cycle 11, July to December 2004. Canberra: Report to the Australian Government Department of Health and Ageing, Diagnostics and Technology Branch, 2004.
- 15. Cagliero E, Levina EV, Nathan DM. Immediate feedback of HbA1c levels improves glycemic control in type 1 and insulin-treated type 2 diabetes. Diabetes Care 1999; 22: 1785-1789.
- 16. Miller CD, Barnes CS, Phillips LS, et al. Rapid A1c availability improves clinical decision-making in an urban primary care clinic. Diabetes Care 2003; 26: 1158-1163.
- 17. Simmons D. Impact of an integrated approach to diabetes care at the Rumbalara Aboriginal Health Service. Intern Med J 2003; 33: 581-585.





Abstract
Objectives: To assess the accuracy of point-of-care (POC) measurements of capillary blood glucose and glycosylated haemoglobin (HbA1c) levels in a remote Aboriginal community with high diabetes prevalence.
Design: Cross-sectional study comparing POC capillary glucose and HbA1c results with those from corresponding venous samples measured in a reference laboratory.
Participants and setting: 152 residents aged 11–76 years (representing 76% of population aged over 11 years) had POC glucose measurement in November 2003; 88 with POC glucose level ≥ 5.0 mmol/L, or self-reported diabetes, had POC HbA1c and laboratory glucose and HbA1c measurements.
Main outcome measures: POC fasting capillary levels of glucose (HemoCue Glucose 201 analyser, Medipac Scientific, Sydney) and HbA1c (DCA 2000+ analyser, Bayer Australia, Melbourne); correlation and mean difference between capillary POC and venous blood laboratory measurements of glucose and HbA1c.
Results: Mean and median POC capillary glucose levels were 7.99 mmol/L and 6.25 mmol/L, respectively, while mean and median laboratory venous plasma glucose concentrations were 7.63 mmol/L and 5.35 mmol/L. Values for POC capillary HbA1c and laboratory HbA1c were identical: mean, 7.06%; and median, 6.0%. The correlation coefficient r for POC and laboratory results was 0.98 for glucose and 0.99 for HbA1c. The mean difference in results was 0.36 mmol/L for glucose (95% CI, 0.13–0.62; limits of agreement [LOA], − 2.07 to 2.79 mmol/L; P = 0.007) and < 0.01% for HbA1c (95% CI, − 0.07% to 0.07%; LOA, − 0.66% to 0.66%; P = 0.95), respectively.
Conclusions: POC capillary HbA1c testing, in particular, offers an accurate, practical, community-friendly way of monitoring diabetes in rural and remote clinical settings. POC capillary glucose results should be confirmed by a laboratory test of venous plasma if the results are likely to significantly influence clinical decisions.