The known: The burden of rheumatic heart disease in Aboriginal and Torres Strait Islander people is high, and many patients present with late complications of the disease and without a history of acute rheumatic fever.
The new: Echocardiographic screening in the remote Northern Territory town of Maningrida detected a large burden of undiagnosed rheumatic heart disease in young Aboriginal Australians. The total prevalence of rheumatic fever and rheumatic heart disease among people aged 5–20 years was at least 10%.
The implications: Echocardiographic screening for active case finding is needed to improve health outcomes for people living in remote Australian communities with a high burden of rheumatic heart disease.
The rates of acute rheumatic fever (ARF; 354 per 100 000 people in 2017) and rheumatic heart disease (RHD; 2436 per 100 000 people) for Aboriginal and Torres Strait Islander people in the Northern Territory are among the highest in the world; the RHD rate is much higher than for non‐Indigenous Territorians (63 per 100 000 people).1 Prevalence is highest in remote communities where inadequate housing leads to household crowding,2 markedly increasing the risk of group A streptococcal infections.3
In 1997, an RHD control program was established in the NT to facilitate collection of epidemiologic data based on ARF and RHD notifications and to coordinate medical care, including secondary prophylaxis.4 In 2017, about 5% of NT Indigenous Australians were registered for secondary prophylaxis (4‐weekly intramuscular benzathine benzylpenicillin G injections) because they had been diagnosed with ARF or RHD.1
In general, most patients with RHD do not have documented histories of ARF, and they present with late complications, such as heart failure, arrhythmia, stroke, endocarditis, or pregnancy‐related complications.5 The absence of a documented history of ARF is more common in low (78% of people with RHD) and lower middle income countries (56%) than in upper middle income countries (41%).5 Even in Australia, where the health system is well resourced and health care freely available to all residents, people often first present with RHD at an advanced stage of the disease. In the NT, 14% of people with RHD were first diagnosed when they presented with heart failure.4
Echocardiographic screening according to World Heart Federation criteria6 is the most reliable way of detecting RHD in asymptomatic people, and it can identify large numbers of previously undetected cases.7,8,9 Echocardiographic screening of school‐age children in the Top End of the NT indicated that the prevalence of RHD was 15 cases per 1000 children; about half the detected cases of RHD had not previously been diagnosed.10
People with RHD detected by screening can receive appropriate cardiac care, including guideline‐recommended secondary prophylaxis.11 This may be a cost‐effective approach to managing RHD in groups at high risk in northern Australia.12 Nevertheless, large scale screening has not been undertaken, and studies are underway to determine the best models for sustainable targeted screening and to evaluate the impact of screening and treatment on clinical outcomes.13
In this study, we aimed to determine the role of echocardiographic screening for detecting undiagnosed RHD, and to estimate the prevalence of ARF and RHD in Maningrida, a remote NT town with a high burden of RHD.
Methods
Design
We conducted a prospective cross‐sectional echocardiographic screening study in Maningrida. We then combined our screening findings with data from the NT RHD register to describe the burdens of ARF and RHD among people in Maningrida aged 5–20 years.
Setting and community involvement in study design
Maningrida, located in West Arnhem Land (Box 1), has a population of 2366 Indigenous Australian people and 244 non‐Indigenous Australians (2016).14 In 2014, a cluster of ARF cases was detected against a background of high baseline incidence, based on notifications to the NT RHD register.15 Community members recognised the importance of RHD, and teachers at the only school in Maningrida reported that many students received regular benzathine benzylpenicillin G injections. Following extensive discussions with local people, a collaboration between clinicians, researchers, traditional owners, community leaders, clinic staff, and teachers developed a strategy for raising awareness of RHD, dealing with primary risk factors, promoting recognition and treatment of group A streptococcal infections, improving adherence by people with RHD to treatment, and identifying new cases in Maningrida.
At the invitation of community members, we worked with the Mala'la Health Board, the Maningrida Health Centre, the Bawinanga Aboriginal Corporation, West Arnhem Regional Council, Maningrida College, and the Lurra Language and Culture Unit to develop a community response to ARF and RHD. A curriculum of learning objectives and activities regarding germ theory, group A streptococcal infections, and ARF and RHD was developed for school students in local languages (Njébbana, Burarra, Kuninjku, Djinang) by the Lurra Language and Culture Unit. Echocardiographic screening followed the six‐week education program. Screening was undertaken in the only school, at the local swimming pool, and in remote homelands to maximise coverage.
Procedures
Children and young adults aged 5–20 years living in Maningrida and the surrounding homelands were invited to participate in echocardiographic screening for RHD in March and November 2018. Informed consent was obtained in writing from participants or their legal guardians after providing explanations of the study in local languages. One of three cardiologists (two were paediatric cardiologists) or one of four cardiac sonographers with experience in the diagnosis of RHD performed echocardiography screening with a Vivid Q or Vivid I portable ultrasound machine (GE Healthcare). Echocardiographic screening and diagnostic protocols were based on World Heart Federation guidelines,5 and included parasternal long and short axis and apical four‐ and five‐chamber views (2D and Doppler). People with abnormal echocardiograms underwent full anatomic scans to exclude congenital heart disease.
To improve the reliability of RHD diagnoses, all abnormal echocardiograms were reviewed on site by a panel of three expert cardiologists or sonographers, who reached a final diagnosis by consensus.7,16 RHD was classified as borderline or definite according to World Heart Federation criteria (Box 2), and graded for severity according to Australian guidelines.5,10
We checked the NT RHD register and the clinical database of NT Cardiac (a private organisation that is the main provider of specialist cardiac services in the Top End) for histories of ARF or RHD for all participants who had provided consent. In addition, aggregate numbers of notified ARF and RHD cases for Maningrida were obtained from the NT RHD register in November 2018. ARF diagnoses conformed with the then current 2012 Australian guidelines (Box 2).10
Data management and analysis
Data were collected in a REDCap 8.7.4 (Vanderbilt University) database hosted at the Menzies School of Health Research, Darwin. Statistical analysis was conducted in Stata 15.1. The prevalence of RHD was calculated (with Clopper–Pearson 95% confidence intervals, CIs). The influence of sex and age group on prevalence was expressed as odds ratios derived from univariate analyses and adjusted odds ratios derived from multivariate analyses (logistic regression). Indigenous status was not included as a factor in multivariate analyses because all participants with RHD were Aboriginal Australians. P < 0.05 was deemed statistically significant.
Ethics approval
Ethics approval was obtained from the Human Research Ethics Committee of the NT Department of Health and Menzies School of Health Research (reference, 17‐3011) and the Aboriginal Ethics Committee of the Menzies School of Health Research. Traditional owners, elders, and other Aboriginal leaders from Maningrida requested that the results of the study be published, and that Maningrida be named in all publications.
Results
An estimated 849 people aged 5–20 years lived in Maningrida during the study period; 166 could not be contacted at the time of the study. Consent was obtained for 676 people (80%), of whom 613 (91%) attended echocardiography screening (Box 3). The median age of the screened participants was 11 years (interquartile range, 8–14 years); 298 (49%) were girls or women, and 592 (97%) identified as Aboriginal Australians.
Prior to our study, 156 participants (25%) had undergone echocardiography for clinical reasons. Twelve (2%) had previous diagnoses of definite RHD, including three who had had surgery for severe RHD. A further 25 participants (4%) had histories of ARF without cardiac involvement.
At screening, twenty participants (3.3%) were diagnosed with previously unknown definite RHD and 14 (2.3%) with borderline RHD. In total, 32 participants (5.2%) had definite RHD and 17 (2.8%) borderline RHD; all were Aboriginal Australians (Box 3, Box 4). Nine of 32 participants with definite RHD (28%) had documented histories of ARF.
The proportions of male (4.1%) and female participants (6.4%) with definite RHD were similar. By age group, prevalence was greatest for participants aged 16–20 years (10 of 104 participants, 9.6%) (Box 5), but the proportion of previously undiagnosed RHD was greatest for participants aged 10–15 years (15 of 263, 5.7%).
The prevalence of definite or borderline RHD among the 592 screened Aboriginal people aged 5–20 years was 8.3% (95% CI, 6.3–11%; 49 participants); it was 5.4% (95% CI, 3.8–7.6%; 32 participants) for definite RHD and 2.9% (95% CI, 1.8–4.6%; 17 participants) for borderline RHD. The prevalence of severe definite RHD was 1% (95% CI, 0.7–3%; eight participants).
Clinically significant non‐RHD cardiac disease was identified in 11 of the 613 screened participants (1.8%), of whom four had histories of surgery for congenital heart disease and seven of newly diagnosed clinically significant heart disease (two with patent ductus arteriosus, both referred for device closure; two with bicuspid aortic valve; two with congenital mitral valve prolapse; one with dilated cardiomyopathy). A further nine participants had evidence of minor congenital heart disease that was not clinically significant.
All new cases of definite and borderline RHD identified in our screening study were notified to the NT RHD register and the affected participants were provided education in local languages, including information about secondary prophylaxis and monitoring for symptoms of group A streptococcal infection and ARF. Secondary prophylaxis (benzathine benzylpenicillin G) was initiated within a day of a new diagnosis of definite RHD. All participants with definite or borderline RHD were referred to cardiac services for follow‐up. Of the five participants with newly identified severe RHD, three had mitral valve surgery within six months of their diagnosis. According to NT RHD register data at the end of the study (November 2018), 88 of 849 people aged 5–20 years in Maningrida and the surrounding homelands (10%) were receiving secondary prophylaxis because they had been diagnosed with definite RHD or definite or probable ARF.
Discussion
The estimated prevalence of RHD among young people in Maningrida in our echocardiographic screening study — 5.4 definite cases per 100 people — is the highest that has been reported for any population.9,10,17,18 A 2017 review of the global burden of RHD included only 20 countries with an age‐standardised prevalence of RHD that exceeded 1%.17 This emphasises the scale of the problem in Maningrida, where more than 1% of Aboriginal children have had cardiac surgery for severe RHD.
This high burden of RHD will have substantial impact on individual morbidity and mortality, as well as upon health services in Maningrida. More than 10% of people living in Maningrida aged 5–20 years are currently prescribed secondary prophylaxis because they have RHD or a history of ARF. Outcomes for Indigenous people with RHD are frequently poor, including heart failure, cerebrovascular accidents, and premature death; about 10% of those with severe RHD at the time of diagnosis die within 6 years.19 Ten‐year mortality for 79 children (including 74 Indigenous patients) who underwent cardiac surgery for RHD in Melbourne was 14%.20
The motivation for active RHD case finding is the fact that initiating secondary prophylaxis can avert further ARF episodes and, with good adherence to treatment, facilitate recovery from valvulitis and the amelioration of RHD.21 Detecting severe RHD also enables early access to life‐saving cardiac surgery, which is associated with more durable valve repair and improved long term survival.20,22 We provided education and follow‐up, but not secondary prophylaxis, to participants with borderline RHD, consistent with international guidelines.6 A randomised controlled trial is currently underway to determine the value of secondary prophylaxis for patients with borderline RHD.13
Economic analyses of modelled clinical outcomes suggest that echocardiographic screening would be cost‐effective in areas of high RHD burden in Australia.12 However, routine echocardiographic screening for early detection has not been widely adopted because of uncertainty about the management of borderline RHD — specifically, whether secondary prophylaxis is appropriate — and reservations about the level of sustained resourcing required.23 Echocardiographic screening strategies that might be more efficient include training non‐expert screening personnel, using abbreviated echocardiography protocols and less expensive, more portable handheld devices, and targeting age groups at greatest risk of undiagnosed RHD. We are currently undertaking research focused on establishing cost‐effective approaches to echocardiographic screening in remote communities.
Auscultation is still used in the NT during the annual screening of school‐aged children in remote communities, despite the low sensitivity and specificity of this approach.8 Children with murmurs detected by clinical examination are routinely referred for echocardiography, as are those with suspected ARF. About one‐quarter of the participants in our study had previously undergone echocardiography. Only 28% of those with RHD had a documented episode of ARF; subclinical RHD can develop without diagnosed ARF, but this is more common in low income countries.5
Our findings suggest that regular auscultation and passive case finding for ARF and RHD may be inadequate in some parts of Australia, exposing children and young people with undetected RHD to the risk of poor health outcomes.
Communication problems and other barriers to culturally safe health care in remote communities, where most people speak languages other than English, have contributed to poorer health outcomes for Aboriginal and Torres Strait Islander people.24,25 To overcome communication barriers, we delivered education in local languages and using local metaphors. Involving traditional owners and members of the West Arnhem Regional Council was a key feature of our study, and local leadership continues to drive community‐led initiatives for tackling the now well understood burden of RHD. Community leadership in designing and implementing RHD screening must be ensured.
Limitations
We screened only 72% of the target population, but this was better than anticipated given local school attendance levels. Young people who were not enrolled in the study may be less engaged with health care, and including them may have increased our estimated prevalence of RHD. We continue to try to reach these young people. Our study was undertaken at a single site, and our findings cannot be extrapolated to other parts of Australia. A further limitation is the reliability of diagnosis and of severity categorisation for definite and borderline RHD.7 However, we adhered to established guidelines, and categorisation was based on consensus among three experts.6,16
Conclusion
Our study illustrates the importance of echocardiographic screening for RHD in a remote Australian town where, despite an established territorial RHD register and access to free health care, an unprecedented burden of undiagnosed RHD was detected. In some settings, echocardiographic screening should be considered, together with primordial and primary prevention activities, as part of a comprehensive approach to eliminating RHD. Community leadership and participation in screening will increase the impact of such programs, and the factors that facilitate community‐led responses to problems such as RHD should be examined. In Australia, we recommend sharing notifiable disease data and register information at the community level, so that communities with higher burdens of ARF and RHD can receive priority support for local responses, which should include support for active case finding of RHD by echocardiographic screening.
Box 2 – Acute rheumatic fever and rheumatic heart disease: definitions
|
|
|||||||||||||||
|
Australian guidelines for the diagnosis of acute rheumatic fever in groups at high risk (2015) 11 |
|||||||||||||||
Definite acute rheumatic fever
|
|||||||||||||||
Probable acute rheumatic fever
|
|||||||||||||||
Major criteria
|
|||||||||||||||
Minor criteria
|
|||||||||||||||
|
World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease (RHD) in people aged 20 years or less (2012) 6 |
|||||||||||||||
Definite rheumatic heart disease (any of four criteria)
|
|||||||||||||||
Borderline rheumatic heart disease (any of three criteria)
|
|||||||||||||||
|
|
|||||||||||||||
|
* Congenital mitral valve anomalies must be excluded. † Bicuspid aortic valve, dilated aortic root, and hypertension must be excluded. ‡ Combined aortic and mitral valve regurgitation in people without congenital heart disease in geographic regions of high prevalence is regarded as rheumatic. |
|||||||||||||||
Box 3 – Selection and evaluation of participants for electrocardiographic screening
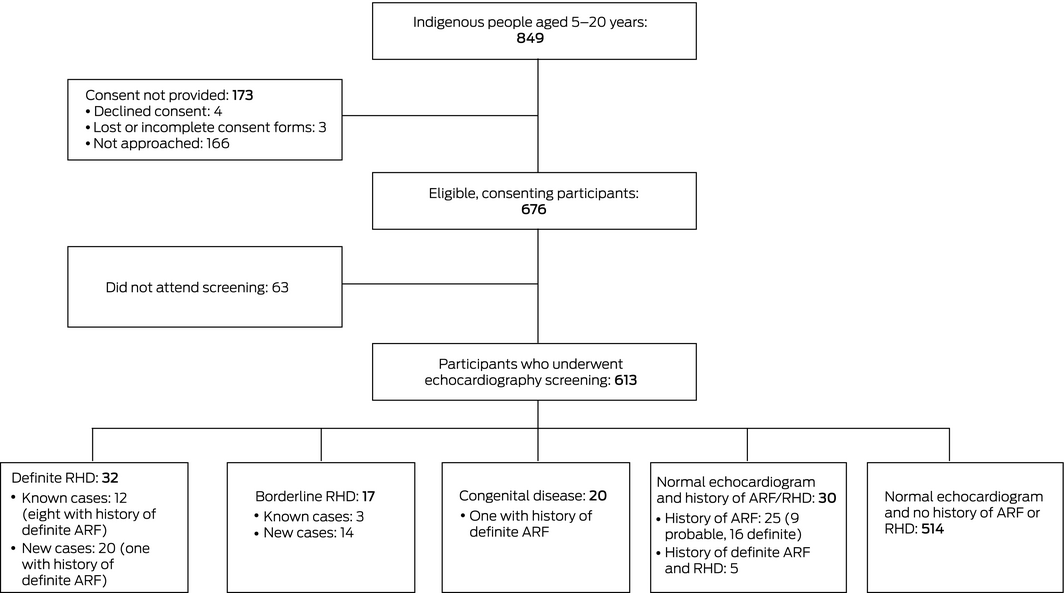
ARF = acute rheumatic fever; RHD = rheumatic heart disease.
Box 4 – Rheumatic heart disease detected during screening of 613 Indigenous Australians aged 5–20 years in Maningrida, 2018
|
Diagnosis |
Cases of rheumatic heart disease |
||||||||||||||
|
Total number |
New cases |
Previously identified |
|||||||||||||
|
|
|||||||||||||||
|
Borderline rheumatic heart disease |
17 |
14 |
3 |
||||||||||||
|
Mild mitral regurgitation |
7 |
7 |
0 |
||||||||||||
|
Mild aortic regurgitation |
7 |
5 |
2 |
||||||||||||
|
Pathologic valve changes |
3 |
2 |
1 |
||||||||||||
|
Definite rheumatic heart disease |
32 |
20 |
12 |
||||||||||||
|
Mild rheumatic heart disease |
12 |
8 |
4 |
||||||||||||
|
Mild mitral regurgitation |
10 |
6 |
4 |
||||||||||||
|
Mild aortic regurgitation |
2 |
2 |
0 |
||||||||||||
|
Moderate rheumatic heart disease |
12 |
7 |
5 |
||||||||||||
|
Moderate mitral regurgitation |
6 |
3 |
3 |
||||||||||||
|
Mild mitral regurgitation and mild aortic regurgitation |
3 |
3 |
0 |
||||||||||||
|
Moderate mitral regurgitation and moderate aortic regurgitation |
1 |
0 |
1 |
||||||||||||
|
Moderate mitral regurgitation and mild aortic regurgitation |
2 |
1 |
1 |
||||||||||||
|
Severe rheumatic heart disease |
8 |
5* |
3† |
||||||||||||
|
Severe mitral regurgitation |
5 |
5 |
0 |
||||||||||||
|
Post‐surgical repair |
3 |
0 |
3 |
||||||||||||
|
|
|||||||||||||||
|
* Three of these participants subsequently had surgery (total of four operations). † The three participants had had surgery before the study (total of five operations). |
|||||||||||||||
Box 5 – Prevalence of definite rheumatic heart disease among Indigenous Australians in Maningrida aged 5–20 years, 2018: univariate and multivariate analyses
|
Characteristic |
|
Univariate analysis |
Multivariate analysis |
||||||||||||
|
Number |
Odds ratio (95% CI) |
Adjusted odds ratio* (95% CI) |
|||||||||||||
|
|
|||||||||||||||
|
Total number of cases |
32/613 (5.2%) |
|
|
||||||||||||
|
Sex |
|
|
|
||||||||||||
|
Male |
13/315 (4.1%) |
1 |
1 |
||||||||||||
|
Female |
19/298 (6.4%) |
1.6 (0.7–3.5) |
1.5 (0.7–3.1) |
||||||||||||
|
Age (years) |
|
|
|
||||||||||||
|
5–9 |
4/246 (2%) |
1 |
1 |
||||||||||||
|
10–15 |
18/263 (6.8%) |
4.4 (1.5–13) |
4.4 (1.5–13) |
||||||||||||
|
16–20 |
10/104 (9.6%) |
6.4 (2.0–21) |
6.3 (1.9–20) |
||||||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Adjusted for age or sex. |
|||||||||||||||
Received 15 September 2019, accepted 23 April 2020
- Joshua R Francis1,2
- Helen Fairhurst1
- Hilary Hardefeldt2
- Shannon Brown3
- Chelsea Ryan3
- Kurt Brown3
- Greg Smith3
- Roz Baartz3
- Ari Horton2
- Gillian Whalley4
- James Marangou5
- Alex Kaethner5
- Anthony DK Draper6
- Christian L James6
- Alice G Mitchell1
- Jennifer Yan1,2
- Anna Ralph1
- Bo Remenyi2,5
- 1 Menzies School of Health Research, Charles Darwin University, Darwin, NT
- 2 Royal Darwin Hospital, Darwin, NT
- 3 Top End Health Service, Maningrida Health Centre, Maningrida, NT
- 4 University of Otago, Dunedin, New Zealand
- 5 NT Cardiac, Darwin, NT
- 6 Centre for Disease Control, Northern Territory Department of Health, Darwin, NT
The Pedrino Project (early detection and treatment of rheumatic heart disease in high risk communities using community‐led approaches) is a Menzies School of Health Research project, supported by a Heart Foundation Vanguard Grant and a pilot grant from the National Health and Medical Research Council (1131932: Improving health outcomes in the tropical north: a multidisciplinary collaboration [HOT NORTH]). It was also supported by Rotary Oceania Medical Aid for Children (ROMAC), the Snow Foundation, the Bawinanga Aboriginal Corporation, and the Humpty Dumpty Foundation, and by in‐kind support from NT Cardiac, the Starlight Children's Foundation, Take Heart (Moonshine Agency), the Northern Territory Department of Health, the Maningrida Health Centre, the Northern Territory Rheumatic Heart Disease Control program, the Mala'la Health Board, Maningrida College, the Lurra Language and Culture Unit, and the West Arnhem Regional Council. Anna Ralph is supported by a National Health and Medical Research Council fellowship (1142011).We acknowledge the contributions of Leroy Bading, Lionel Cooper, Madeline Mackey, Lachlan Nicolson, Erin Riddell and Matthew Ryan (West Arnhem Shire Council, logistics); Joyce Bohme, Roderick Brown and Rickisha Redford Bohme (Bawinanga Aboriginal Corporation, logistics); Georgina Byron (Snow Foundation, communications); Abigail Carter, Carolyn Coleman, Joseph Diddo, Laurie Guraylayla, Alistair James, Cindy Jinmarabynana, Nelson Nawilmak, Stanley Rankin, Mason Scholes, Russell Stewart and Karen Wuridjal (Maningrida College, education); Sue Collins and Mike Hill (Moonshine Agency, communications); Laura Francis, Kate Hardie, Lorraine Harry, Kristine McConnell‐King, Karen Shergold, Steven Wilson Dashwood and James Woods (Top End Health Services, logistics); Trudy Francis and Kaya Gardiner (data entry); Debbie Hall (Menzies School of Health, logistics); Kate Johnston, Daniel Milne and Sarah Reuben (Starlight Foundation, participant entertainment); Jo Killmister, Daryll Kinnane and Craig Watkins (Maningrida College, logistics); Chris Lowbridge (Menzies School of Health, graphics); Trephrena Taylor and Lesley Woolf (Mala'la Health Board, logistics); and Corinne Toune and Rhiannon Townsend (NT Cardiac, echocardiography).
No relevant disclosures.
- 1. Australian Institute of Health and Welfare. Acute rheumatic fever and rheumatic heart disease in Australia (AIHW Cat. no. CVD 86). Canberra: AIHW, 2019. https://www.aihw.gov.au/reports/cvd/086/acute-rheumatic-fever-rheumatic-heart-disease/contents/introduction/arf-rhd-preventable-diseases (viewed Oct 2019).
- 2. Lowell A, Maypilama Ḻ, Fasoli L, et al. The “invisible homeless”: challenges faced by families bringing up their children in a remote Australian Aboriginal community. BMC Public Health 2018; 18: 1382.
- 3. Coffey PM, Ralph AP, Krause VL. The role of social determinants of health in the risk and prevention of group A streptococcal infection, acute rheumatic fever and rheumatic heart disease: a systematic review. PLoS Negl Trop Dis 2018; 12: e0006577.
- 4. Lawrence JG, Carapetis JR, Griffiths K, et al. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation 2013; 128: 492–501.
- 5. Zühlke L, Engel ME, Karthikeyan G, et al. Characteristics, complications, and gaps in evidence‐based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry (the REMEDY study). Eur Heart J 2015; 36: 1115–1122.
- 6. Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease: an evidence‐based guideline. Nat Rev Cardiol 2012; 9: 297–309.
- 7. Remenyi B, Carapetis J, Stirling JW, et al. Inter‐rater and intra‐rater reliability and agreement of echocardiographic diagnosis of rheumatic heart disease using the World Heart Federation evidence‐based criteria. Heart Asia 2019; 11: e011233.
- 8. Roberts KV, Brown ADH, Maguire GP, et al. Utility of auscultatory screening for detecting rheumatic heart disease in high‐risk children in Australia's Northern Territory. Med J Aust 2013; 199: 196–199. https://www.mja.com.au/journal/2013/199/3/utility-auscultatory-screening-detecting-rheumatic-heart-disease-high-risk
- 9. Rothenbühler M, O'Sullivan CJ, Stortecky S, et al. Active surveillance for rheumatic heart disease in endemic regions: a systematic review and meta‐analysis of prevalence among children and adolescents. Lancet Glob Health 2014; 2: e717–e726.
- 10. Roberts KV, Maguire GP, Brown A, et al. Rheumatic heart disease in Indigenous children in northern Australia: differences in prevalence and the challenges of screening. Med J Aust 2015; 203: 221. https://www.mja.com.au/journal/2015/203/5/rheumatic-heart-disease-indigenous-children-northern-australia-differences
- 11. RHD Australia (ARF/RHD writing group), National Heart Foundation of Australia, Cardiac Society of Australia and New Zealand. The Australian guideline for prevention, diagnosis and management of acute rheumatic fever and rheumatic heart disease (2nd edition). Darwin: Menzies School of Health Research, 2012. https://www.rhdaustralia.org.au/arf-rhd-guideline (viewed Oct 2019).
- 12. Roberts K, Cannon J, Atkinson D, et al. Echocardiographic screening for rheumatic heart disease in Indigenous Australian children: a cost–utility analysis. J Am Heart Assoc 2017; 6: e004515.
- 13. Beaton A, Okello E, Engelman D, et al. Determining the impact of benzathine penicillin G prophylaxis in children with latent rheumatic heart disease (GOAL trial): study protocol for a randomized controlled trial. Am Heart J 2019; 215: 95–105.
- 14. Australian Bureau of Statistics. Maningrida (and outstations). 2016 Census QuickStats. Updated Oct 2017. https://quickstats.censusdata.abs.gov.au/census_services/getproduct/census/2016/quickstat/IARE704003; https://quickstats.censusdata.abs.gov.au/census_services/getproduct/census/2016/quickstat/SSC70172?opendocument (viewed Sept 2019).
- 15. Francis JR, Gargan C, Remenyi B, et al. A cluster of acute rheumatic fever cases among Aboriginal Australians in a remote community with high baseline incidence. Aust N Z J Public Health 2019; 43: 288–293.
- 16. Culliford‐Semmens N, Nicholson R, Tilton E, et al. The World Heart Federation criteria raise the threshold of diagnosis for mild rheumatic heart disease: three reviewers are better than one. Int J Cardiol 2019; 291: 112–118.
- 17. Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional, and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 2017; 377: 713–722.
- 18. Davis K, Remenyi B, Draper ADK, et al. Rheumatic heart disease in Timor‐Leste school students: An echocardiography‐based prevalence study. Med J Aust 2018; 208: 303–307. https://www.mja.com.au/journal/2018/208/7/rheumatic-heart-disease-timor-leste-school-students-echocardiography-based
- 19. Cannon J, Roberts K, Milne C, Carapetis JR. Rheumatic heart disease severity, progression and outcomes: a multi‐state model. J Am Heart Assoc 2017; 6: e004515.
- 20. McGurty D, Remenyi B, Cheung M, et al. Outcomes after rheumatic mitral valve repair in children. Ann Thorac Surg 2019; 108: 792–797.
- 21. de Dassel JL, de Klerk N, Carapetis JR, Ralph AP. How many doses make a difference? An analysis of secondary prevention of rheumatic fever and rheumatic heart disease. J Am Heart Assoc 2018; 7: e010223.
- 22. Remenyi B, Webb R, Gentles T, et al. Improved long‐term survival for rheumatic mitral valve repair in compared to replacement in the young. World J Pediatr Congenit Hear Surg 2013; 4: 155–164.
- 23. Roberts K, Colquhoun S, Steer A, et al. Screening for rheumatic heart disease: current approaches and controversies. Nat Rev Cardiol 2013; 10: 49–58.
- 24. Walsh WF, Kangaharan N. Cardiac care for indigenous Australians: practical considerations from a clinical perspective. Med J Aust 2017; 207: 40–45. https://www.mja.com.au/journal/2017/207/1/cardiac-care-indigenous-australians-practical-considerations-clinical
- 25. Amery R. Recognising the communication gap in Indigenous health care. Med J Aust 2017; 207: 13–15. https://www.mja.com.au/journal/2017/207/1/recognising-communication-gap-indigenous-health-care





Abstract
Objectives: Using echocardiographic screening, to estimate the prevalence of rheumatic heart disease (RHD) in a remote Northern Territory town.
Design: Prospective, cross‐sectional echocardiographic screening study; results compared with data from the NT rheumatic heart disease register.
Setting, participants: People aged 5–20 years living in Maningrida, West Arnhem Land (population, 2610, including 2366 Indigenous Australians), March 2018 and November 2018.
Intervention: Echocardiographic screening for RHD by an expert cardiologist or cardiac sonographer.
Main outcome measures: Definite or borderline RHD, based on World Heart Federation criteria; history of acute rheumatic fever (ARF), based on Australian guidelines for diagnosing ARF.
Results: The screening participation rate was 72%. The median age of the 613 participants was 11 years (interquartile range, 8–14 years); 298 (49%) were girls or women, and 592 (97%) were Aboriginal Australians. Definite RHD was detected in 32 screened participants (5.2%), including 20 not previously diagnosed with RHD; in five new cases, RHD was classified as severe, and three of the participants involved required cardiac surgery. Borderline RHD was diagnosed in 17 participants (2.8%). According to NT RHD register data at the end of the study period, 88 of 849 people in Maningrida and the surrounding homelands aged 5–20 years (10%) were receiving secondary prophylaxis following diagnoses of definite RHD or definite or probable ARF.
Conclusion: Passive case finding for ARF and RHD is inadequate in some remote Australian communities with a very high burden of RHD, placing children and young people with undetected RHD at great risk of poor health outcomes. Active case finding by regular echocardiographic screening is required in such areas.