Iodine is an essential micronutrient required for thyroid hormone synthesis. Inadequate dietary iodine intake is associated with a spectrum of diseases termed iodine deficiency disorders. The most serious and overt consequences are neurocognitive disorders and endemic goitre.1 Urinary iodine excretion is a marker of recent dietary iodine intake and is typically used to monitor population iodine sufficiency. Population iodine status is considered optimal when median urinary iodine concentration (UIC) is between 100 µg/L and 199 µg/L, with no more than 20% of samples having UIC under 50 µg/L.1
Concern about the emergence of widespread mild iodine deficiency in Australia and New Zealand led to mandatory iodine fortification of yeast-leavened bread in 2009.2 Tasmania has a well documented history of endemic iodine deficiency, with iodine supplementation strategies implemented since the 1950s.3 The use of iodophors as sanitising agents in the dairy industry was thought to have provided protection; however, urinary iodine surveys of Tasmanian school children in 1998 and 2000 showed a recurrence of iodine deficiency.4
In October 2001, the Tasmanian Government introduced a state-based voluntary iodine fortification program as an interim measure to reduce the recurrence of iodine deficiency. This program resulted in a modest but significant improvement in population iodine status.5 The Tasmanian voluntary fortification experience provided valuable information for the development of the Australia and New Zealand mandatory iodine fortification program.
A cross-sectional urinary iodine survey of Tasmanian schoolchildren was conducted in 2011. Survey methods were comparable to those used during the period of voluntary fortification, as described elsewhere.5
A one-stage cluster sampling method was used to randomly select school classes that included fourth-grade students from all government, Catholic and independent schools in Tasmania (such classes may include children in third, fourth, fifth and sixth grade, as composite class structures are popular in Tasmania). A total of 52 classes (from 49 schools) were invited to participate. This included 42 classes that had been randomly selected for the final survey conducted during the period of voluntary fortification and an additional 10 classes randomly selected in 2011 to boost sample size. In total, 37 classes (from 35 schools) agreed to take part, representing a class participation rate of 71%. Of the 880 children in participating classes, 356 (40%) returned positive consent and 320 (36%) provided a urine sample for analysis. These participation rates are comparable with the rates reported from previous surveys.5
Spot urine samples were collected at home, returned to school and transported by a private pathology provider to a laboratory where they were frozen and stored. Batch analyses were completed by the Institute of Clinical Pathology and Medical Research, Westmead Hospital. UIC was measured using the ammonium persulfate digestion method based on the Sandell–Kolthoff reaction.6
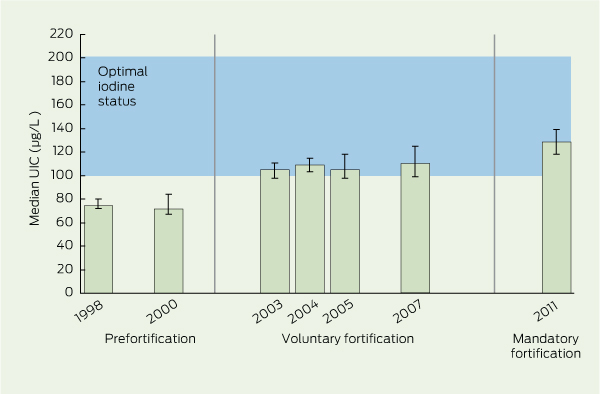
The median UIC in 2011 was significantly higher than during the period of voluntary fortification (129 µg/L v 108 µg/L; P < 0.001), which in turn was significantly higher than the median UIC from the prefortification period (73 µg/L; P < 0.001) (Box 1). There was a reduction in the proportion of UIC results under 50 µg/L after voluntary fortification compared with prefortification, from 17.7% to 9.6% (P < 0.001), and a further reduction to 3.4% after mandatory fortification (P = 0.001) (Box 2). Box 3 shows the progressive improvement in median UIC results from Tasmanian urinary iodine surveys of schoolchildren over the iodine fortification intervention periods (prefortification, voluntary fortification and mandatory fortification).
Population iodine status is routinely assessed by measuring UIC, whereas determining the appropriate level of fortification in food relies on estimates of dietary intakes. The relationship between dietary iodine intake and UIC is usually linear — an increase in dietary intake results in a comparable increase in urinary excretion.7 The 56 µg/L increase in median UIC from prefortification to mandatory fortification is consistent with the predicted 52 µg/d increase in the mean dietary iodine intake for children aged 9–13 years, estimated by dietary modelling before the introduction of mandatory iodine fortification.8
In the 2004 National Iodine Nutrition Study, a survey of schoolchildren found that Western Australia had the highest median UIC of all Australian jurisdictions, at 142.5 µg/L.9 Extrapolating the magnitude of increase in UIC from our surveys to that observed in WA would result in a UIC just under 200 µg/L (56 µg/L + 142 µg/L), which is at the upper level of the optimal range.1
To facilitate comparisons, the sampling method used in our 2011 survey was modelled on the method used in the surveys conducted during the period of voluntary fortification.5 Classes that included fourth-grade children were originally chosen as the sampling frame to be consistent with World Health Organization guidelines for assessing population iodine status.1 Staff from the Department of Education Tasmania advised that this age group would be sufficiently independent to provide a urine sample, while minimising self-consciousness likely in older children. It is yet to be seen whether the observed impact of mandatory fortification is representative of other population groups, such as adults. Published surveys of prefortification UIC of Melbourne adults offer a useful baseline for this purpose.10 The Australian Health Survey 2011–2013 is measuring UIC in adults and children across Australia, and we anticipate this will provide further evidence of the iodine status in the Australian population.
Comparisons with prefortification surveys should be interpreted with the knowledge that there were subtle differences in sampling methods. A two-stage stratified sampling procedure was adopted in the prefortification period (1998–2000), where schools and then students from within schools were randomly selected. Subsequent surveys used a one-stage cluster sampling method with classes that included fourth-grade students as the sampling frame. These sampling differences are not considered significant and have been discussed elsewhere.5 Any sample bias associated with factors such as socioeconomic status or geographic location is unlikely to affect the results, as an association between UIC and these factors has not been found previously.4
Although the 2011 results are consistent with iodine repletion in the general population, they cannot be generalised to high-risk subgroups such as pregnant and breastfeeding women, whose daily iodine requirements increase by about 40%.11 Prior research in Tasmania has shown persistent iodine deficiency in pregnancy despite the introduction of voluntary iodine fortification.12 Recent evidence suggests that while mandatory iodine fortification may have benefited breastfeeding women, only those consuming iodine-containing supplements had a median UIC in the adequate range.13 Future studies of iodine nutrition should specifically assess the adequacy in these groups. Similarly, ongoing awareness of the recommendation that pregnant and lactating women take 150 µg of supplemental iodine per day should not be overlooked, particularly in those parts of Australia where marginal iodine deficiency has been previously reported.14,15
Changes to the iodine content of food supply (such as the level of iodine in milk or the level of salt in bread) or shifts in dietary choice (such as a preference for staples other than bread) could jeopardise iodine status in the future.3,16 The value of ongoing vigilance in monitoring population iodine status has been highlighted by previous authors.12,17,18 In addition, monitoring iodine levels in the food supply will be required to inform future adjustments to the mandatory iodine fortification program.
2 Comparison of urinary iodine concentration (UIC) of Tasmanian schoolchildren across intervention periods
Received 5 September 2012, accepted 1 April 2013
- Kate M DePaoli1
- Judy A Seal1
- John R Burgess2,3
- Roscoe Taylor1
- 1 Population Health, Department of Health and Human Services, Hobart, TAS.
- 2 School of Medicine, Faculty of Health Science, University of Tasmania, Hobart, TAS.
- 3 Royal Hobart Hospital, Hobart, TAS.
Permission was granted by the Tasmanian Department of Health and Human Services to use the 2011 urinary iodine survey data. Statistical analysis was undertaken by Michael Long. We are grateful to the school staff, parents, carers and children who participated in the urinary iodine surveys.
No relevant disclosures.
- 1. International Council for Control of Iodine Deficiency Disorders, United Nations Children’s Fund, World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination. 2nd ed. Geneva: WHO, 2001. http://www.who.int/nutrition/publications/micronutrients/iodine_deficiency/WHO_NHD_01.1/en/ (accessed Nov 2010).
- 2. Food Standards Australia New Zealand. Australia New Zealand Food Standards Code – Standard 2.1.1 – Cereals and cereal products. Canberra: FSANZ, 2009. http://www.comlaw.gov.au/Details/F2009C00811 (accessed Jul 2012).
- 3. Gibson HB. Surveillance of iodine deficiency disorders in Tasmania 1949–1984. 2nd ed. Launceston: Myola House of Publishing, 2006.
- 4. Hynes KL, Blizzard CL, Venn AJ, et al. Persistent iodine deficiency in a cohort of Tasmanian school children: associations with socio-economic status, geographical location and dietary factors. Aust N Z J Public Health 2004; 28: 476-481.
- 5. Seal JA, Doyle Z, Burgess JR, et al. Iodine status of Tasmanians following voluntary fortification of bread with iodine. Med J Aust 2007; 186: 69-71. <MJA full text>
- 6. Ohashi T, Yamaki M, Pandav CS, et al. Simple microplate methods for determination of urinary iodine. Clin Chem 2000; 46: 529-536.
- 7. Food Standards Australia New Zealand. Draft assessment report. Proposal P230: consideration of mandatory fortification with iodine. Canberra: FSANZ, 2006. http://www.foodstandards.gov.au/foodstandards/proposals/proposalp230iodinefo2802.cfm (accessed Jun 2012).
- 8. Food Standards Australia New Zealand. Consideration of mandatory fortification with iodine for Australia and New Zealand: dietary intake assessment report. Main report. Canberra: FSANZ, 2008. http://www.foodstandards.gov.au/_srcfiles/P1003%20SD10%20-%20Dietary%20Intake%20Assessment.pdf (accessed Jan 2012).
- 9. Li M, Eastman CJ, Waite KV, et al. Are Australian children iodine deficient? Results of the Australian National Iodine Nutrition Study. Med J Aust 2006; 184: 165-169. <MJA full text>
- 10. Baxter JR, Riddell L, Huggins CE, et al. Iodine status in Melbourne adults in the early 1990s and 2007-08. Aust N Z J Public Health 2011; 35: 408-411.
- 11. National Health and Medical Research Council and New Zealand Ministry of Health. Nutrient reference values for Australia and New Zealand including recommended dietary intakes. Canberra: Commonwealth of Australia, 2006. http://www.nhmrc.gov.au/guidelines/publications/n35-n36-n37 (accessed Apr 2013).
- 12. Burgess JR, Seal JA, Stilwell GM, et al. A case for universal salt iodisation to correct iodine deficiency in pregnancy: another salutary lesson from Tasmania. Med J Aust 2007; 186: 574-576. <MJA full text>
- 13. Axford S, Charlton K, Yeatman H, Ma G. Improved iodine status in breastfeeding women following mandatory iodine fortification. Aust N Z J Public Health 2011; 35: 579-580.
- 14. National Health and Medical Research Council. Iodine supplementation for pregnant and breastfeeding women. NHMRC public statement. Canberra: NHMRC, 2010. http://www.nhmrc.gov.au/guidelines/publications/new45 (accessed Apr 2013).
- 15. Mackerras DE, Eastman CJ. Estimating the iodine supplementation level to recommend for pregnant and breastfeeding women in Australia. Med J Aust 2012; 197: 238-242. <MJA full text>
- 16. Li M, Waite KV, Ma G, Eastman CJ. Declining iodine content of milk and re-emergence of iodine deficiency in Australia [letter]. Med J Aust 2006; 184: 307. <MJA full text>
- 17. Dunn JT. Seven deadly sins in confronting endemic iodine deficiency and how to avoid them. J Clin Endocrinol Metab 1996; 81: 1332-1335.
- 18. Guttikonda K, Burgess JR, Hynes K, et al. Recurrent iodine deficiency in Tasmania, Australia: a salutary lesson in sustainable iodine prophylaxis and its monitoring. J Clin Endocrinol Metab 2002; 87: 2809-2815.





Abstract
Objectives: To examine population iodine status in Tasmania after mandatory iodine fortification of bread and assess the magnitude of difference compared with results from a period of voluntary iodine fortification.
Design and setting: A cross-sectional urinary iodine survey of schoolchildren from classes that included fourth-grade students was conducted in Tasmania in 2011. Results were compared with surveys conducted before fortification and during a period of voluntary fortification.
Participants: Three hundred and twenty students aged 8–13 years from 37 participating school classes.
Main outcome measures: Median urinary iodine concentration (UIC) and proportion of UIC results < 50 µg/L.
Results: Median UIC in 2011 was 129 µg/L, and 3.4% of samples had a UIC under 50µg/L. This was significantly higher than during the period of voluntary fortification (129 µg/L v 108 µg/L) (P < 0.001), which was significantly higher than before fortification (108 µg/L v 73 µg/L) (P < 0.001). There was a reduction in the proportion of samples with UIC under 50 µg/L after mandatory fortification (3.4%) compared with results from the period of voluntary fortification (9.6%) (P = 0.01), which was a further reduction compared with results from the prefortification period (17.7%) (P < 0.001).
Conclusions: Iodine status in Tasmania can now be considered optimal. Mandatory iodine fortification has achieved significantly greater improvements in population iodine status compared with voluntary fortification. However, surveys of schoolchildren cannot be generalised to pregnant and breastfeeding women, who have higher iodine requirements. Measurement of iodine status in population surveys is warranted for ongoing monitoring and to justify the appropriate level of fortification of the food supply into the future.