Iodine deficiency leads to a range of conditions known as iodine deficiency disorders, with the most serious and overt consequences of iodine deficiency — cretinism and widespread goitre — occurring in the context of severe deficiency.1 Mild to moderate iodine deficiency is associated with subclinical anomalies, including neuropsychological dysfunction and deficits in auditory acuity.2,3
Iodine deficiency was prevalent in Tasmania before the 1950s, and strategies to address iodine deficiency were adopted during the 1950s and 1960s.4 Since the early 1970s, iodophors, used as sanitising agents in the dairy industry, are thought to have provided protection against iodine deficiency.
Urinary iodine surveys of Tasmanian schoolchildren in 1998 and 2000 revealed a recurrence of iodine deficiency.5 Reasons postulated for this recurrence include reduced use of iodophors by the dairy industry and reduced household use of iodised salt (caused by a gradual increase in consumption of commercially processed foods containing non-iodised salt).
In October 2001, a voluntary iodine fortification program was implemented in Tasmania. The baking industry was asked to substitute iodised salt for regular salt in bread (details of the program are available from the authors). Mandatory fortification was considered but not pursued, as Tasmania was bound by a national food regulation agreement, which complicated introducing legislative measures at state level. In addition, the program was envisaged as an interim measure; a binational approach was anticipated because of evidence of emerging iodine deficiency elsewhere in Australia and in New Zealand.6-12
Here we present the results from post-intervention urinary iodine surveys of Tasmanian schoolchildren, and compare the results with pre-intervention survey results. Results from pre-intervention surveys and preliminary results from the 2003 post-intervention survey have been published previously.5,13
Cross-sectional urinary iodine surveys of schoolchildren were conducted in 2003, 2004 and 2005. Urinary iodine concentration was determined from spot urine samples using recommended methods.1
Urinary iodine concentration (UIC) was determined by a modified acid digestion method based on the Sandell–Kolthoff reaction.14 The laboratory at which the analysis was done complies with ISO/IEC 17025 under the accreditation scheme of the National Association of Testing Authorities and the Royal College of Pathologists of Australasia.
Data from pre-intervention urinary iodine surveys were extracted for children of the same age range (8–11 years) and used for comparison with the post-intervention surveys. Details of pre-intervention survey methods have been published elsewhere.5
Participation rates in the post-intervention surveys ranged from 37% to 44%. Sample loss occurred through non-return of consent forms (31%–42%), declined consent (19%–20%), and lack of sample collection because the child was absent or unable to produce a sample (2%–6%) (Box 1).
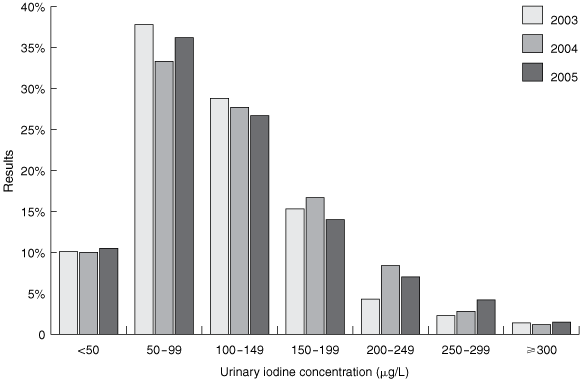
Box 2 shows the distribution of UIC results for the post-intervention surveys. Median UIC was significantly higher in each of the post-intervention years compared with pre-intervention (P < 0.001) (Box 3). There was a reduction in the proportion of UIC results < 50 μg/L in the post-intervention years compared with pre-intervention, but the differences were not significant.
Population iodine status is considered optimal when the median UIC falls between 100 and 199 μg/L, with no more than 20% of samples < 50 μg/L.1 Our post-intervention results are consistent with optimal iodine nutrition, and comparisons with the results from the 1998 pre-intervention survey suggest there has been a modest but significant improvement in median UIC. The observed increase in median UIC is in the order of 30–38 μg/L, which is consistent with increases predicted by dietary modelling before intervention (details can be obtained from the authors).
No attempt was made to measure health outcomes, as the effects of mild iodine deficiency, such as impaired intellectual and physical development, are generally subclinical and likely to be confounded by many factors. Thyroid volume measured by ultrasound is also considered a useful population level indicator of iodine status. However, UIC is considered more sensitive to recent changes in iodine nutrition, as thyroid volume takes time to respond to iodine supplementation.1 Hence, thyroid volume was not measured in our post-intervention surveys.
A two-stage stratified sampling procedure was used in the pre-intervention surveys, compared with one-stage cluster sampling in the post-intervention surveys. Participation rates were lower post-intervention (37%–44%) than in the pre-intervention surveys (> 70%), possibly because ethics approval for the post-intervention surveys did not allow for direct follow-up with parents and carers to encourage participation. This had been standard practice in the pre-intervention surveys. Differences in sampling procedure and response rates are unlikely to have resulted in significant bias associated with factors such as socioeconomic status or geographic location, as prior investigations in Tasmania have not demonstrated an association between UIC and these factors.5
In the post-intervention surveys, casual spot urine samples were collected, compared with first-void samples in the pre-intervention surveys. Both sample types provide an adequate assessment of population iodine status, provided the sample size is large enough.1 However, as UIC follows a circadian rhythm, being lowest first thing in the morning and peaking 4–5 hours after a meal, some researchers recommend that studies restricting sampling time to the morning (first void) should not be directly compared with studies in which urine is sampled throughout the day.15 Although we cannot rule out some influence of the timing of urine sample collection, we believe our results show a real increase in iodine status, as the observed increase in UIC is consistent with predicted levels.
Although no conclusions can be drawn from these data about population subgroups, we remain concerned about groups whose needs may not have been met by this intervention. Such groups include those who consume little or no bread because of special dietary requirements, choice or cultural preferences; those who consume bread predominantly from an outlet not participating in the program; and those with increased iodine requirements. Pregnant and lactating women are of special concern, as their iodine requirements are increased and critical for the growth and development of the fetus and infant.16
As voluntary fortification relies on the goodwill and consistent practices of industry (baking and salt), the reach of the voluntary fortification program may be compromised and there is no guarantee of program sustainability. As iodine deficiency has been confirmed in other parts of Australia,17 and in New Zealand,11 we believe mandatory iodine fortification in both countries would provide a more effective and efficient approach for Tasmania, while addressing iodine deficiency elsewhere.
1 Participation rates in post-intervention surveys of urinary iodine concentration
*Percentages are of total number of children in participating classes. |
|||||||||||||||
Received 10 May 2006, accepted 6 September 2006
- Judy A Seal1
- Zelda Doyle2
- John R Burgess3
- Roscoe Taylor1
- Angus R Cameron2
- 1 Public and Environmental Health Service, Department of Health and Human Services, Hobart, TAS.
- 2 Broadstreet Consultants, Hobart, TAS.
- 3 Endocrinology Laboratory, Royal Hobart Hospital, Hobart, TAS.
Permission was granted by the Tasmanian Department of Health and Human Services to use the urinary iodine survey data. Dr Kristen Hynes, Menzies Centre for Population Health Research, arranged access to the pre-intervention survey data. Statistical advice was provided by Dr Iain Robertson, Clifford Craig Medical Research Trust. Monique Reardon contributed significantly to the design of the Tasmanian Iodine Monitoring Program, to which Prof Creswell Eastman is an expert adviser. Participation by school staff, parents, carers and children in the urinary iodine surveys and the willing cooperation of the Tasmanian baking industry in the voluntary fortification program are gratefully acknowledged.
None identified.
- 1. International Council for Control of Iodine Deficiency Disorders, United Nations Children’s Fund, World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination. 2nd ed. Geneva: WHO, 2001. http://www.who.int/nutrition/publications/micronutrients/en/index.html (accessed Nov 2006).
- 2. Lombardi FA, Pinchera A, Antonangeli L, et al. Mild iodine deficiency during fetal/neonatal life and neuropsychological impairment in Tuscany. J Endocrinol Invest 1995; 18: 57-62.
- 3. Soriguer F, Millon MC, Munoz R, et al. The auditory threshold in a school-age population is related to iodine intake and thyroid function. Thyroid 2000; 10: 991-999.
- 4. Gibson HB. Surveillance of iodine deficiency disorders in Tasmania 1949–1984. 2nd ed. Launceston: Myola House of Publishing, 2006.
- 5. Hynes KL, Blizzard CL, Venn AJ, et al. Persistent iodine deficiency in a cohort of Tasmanian school children: associations with socio-economic status, geographical location and dietary factors. Aust N Z J Public Health 2004; 28: 476-481.
- 6. Gunton JE, Hams G, Fiegert M, McElduff A. Iodine deficiency in ambulatory participants at a Sydney teaching hospital: is Australia truly iodine replete? Med J Aust 1999; 171: 467-470. <MJA full text>
- 7. Li M, Ma G, Boyages SC, Eastman CJ. Re-emergence of iodine deficiency in Australia. Asia Pac J Clin Nutr 2001; 10: 200-203.
- 8. Guttikonda K, Travers CA, Lewis PR, Boyages S. Iodine deficiency in urban primary school children: a cross-sectional analysis. Med J Aust 2003; 179: 346-348. <MJA full text>
- 9. McDonnell CM, Harris M, Zacharin MR. Iodine deficiency and goitre in schoolchildren in Melbourne, 2001. Med J Aust 2003; 178: 159-162. <MJA full text>
- 10. McElduff A, McElduff P, Gunton JE, et al. Neonatal thyroid-stimulating hormone concentrations in northern Sydney: further indications of mild iodine deficiency? Med J Aust 2002; 176: 317-320. <MJA full text>
- 11. Skeaff SA, Thomson CD, Gibson RS. Mild iodine deficiency in a sample of New Zealand schoolchildren. Eur J Clin Nutr 2002; 56: 1169-1175.
- 12. Thomson CD. Selenium and iodine intakes and status in New Zealand and Australia. Br J Nutr 2004; 91: 661-672.
- 13. Seal JA, Johnson EM, Doyle Z, Shaw K. Tasmania: doing its wee bit for iodine nutrition [letter]. Med J Aust 2003; 179: 451-452. <MJA full text>
- 14. Dunn JT, Crutchfield HE, Gutekunst R, Dunn AD. Two simple methods for measuring iodine in urine. Thyroid 1993; 3: 119-123.
- 15. Als C, Helblin A, Peter K, et al. Urinary iodine concentration follows a circadian rhythm: a study with 3023 spot urine samples in adults and children. J Clin Endocrinol Metab 2000; 85: 1367-1369.
- 16. National Health and Medical Research Council, Ministry of Health New Zealand. Nutrient reference values for Australia and New Zealand including recommended dietary intakes. Canberra: NHMRC, 2006. http://www.nhmrc.gov.au/publications/synopses/n35syn.htm (accessed Nov 2006).
- 17. Li M, Eastman CJ, Waite KV, et al. Are Australian children iodine deficient? Results of the Australian National Iodine Nutrition Study. Med J Aust 2006; 184: 165-169. <MJA full text>





Abstract
Objective: To describe changes in the iodine status of Tasmanians following voluntary fortification of bread with iodine in October 2001.
Design and setting: Post-intervention, cross-sectional urinary iodine surveys of Tasmanian schoolchildren aged 8–11 years were used to assess population iodine status. Participants were selected using a one-stage cluster sampling method. The sampling frame comprised classes containing fourth-grade children from all Tasmanian government, Catholic and independent schools. Results were compared with pre-intervention survey results.
Main outcome measures: Median urinary iodine concentration (UIC) and percentage of UIC < 50 μg/L ascertained from spot urine samples.
Results: Median UIC was 75 μg/L in 1998, 72 μg/L in 2000, 105 μg/L in 2003, 109 μg/L in 2004 and 105 μg/L in 2005. Median UIC in post-intervention years (2003–2005) was significantly higher than in pre-intervention years. The percentage of UIC results < 50 μg/L was 16.9% in 1998, 18.7% in 2000, 10.1% in 2003, 10.0% in 2004 and 10.5% in 2005.
Conclusion: Despite methodological differences between the pre- and post-intervention surveys, switching to iodised salt in bread appears to have resulted in a significant improvement in iodine status in Tasmania. Given iodine deficiency has been identified in other parts of Australia and in New Zealand, mandatory iodine fortification of the food supply in both countries is worthy of consideration. As voluntary fortification relies on industry goodwill, mandating fortification could be expected to enhance population reach and give a greater guarantee of sustainability in Tasmania.