Deaths due to non-communicable disease, including chronic conditions, are projected to rise from 59% in 2002 to 69% in 2030.1 In response, the Australian Government developed the National Chronic Disease Strategy (NCDS).2 The NCDS provides an overarching framework to drive the general direction for chronic disease management and National Service Improvement Frameworks for five National Health Priority Areas, including osteoarthritis (Box 1).
Worldwide, about 9.6% of men and 18% of women aged 60 years or older experience symptomatic osteoarthritis.3 These rates are rising concurrently with population ageing and increasing incidence of obesity and trauma.4 About 3.5 million Australians have arthritis, and osteoarthritis is the most prevalent form.5 It is also one of the most common reasons for visiting a general practitioner, with reported rates rising between 1999 to 2006.6
The measurable impacts of osteoarthritis include pain, loss of function, and physical and psychosocial disability. The financial impact is also significant, as increasing rates of joint replacement surgery — a cost-effective intervention for severe osteoarthritis — are a major contributor to rising direct costs of health care.5,7
Many older patients with osteoarthritis require comprehensive assessment because of chronic comorbidities such as obesity, hypertension, cardiac disease, polypharmacy, and use of risk medications such as non-steroidal anti-inflammatory drugs (NSAIDs).8 As with other chronic conditions, symptoms often fluctuate and management can involve multiple health care providers.
Reflecting international guidelines, Australian evidence-based clinical practice guidelines recommend non-pharmacological lifestyle interventions, including physical therapy and weight optimisation, as first-line therapy for osteoarthritis of the hip and knee (Box 2).9,11-13
Use of evidence-based interventions for osteoarthritis results in improved quality of wellbeing, reduced depressed mood,14 improved survival in vulnerable older people,15 and is cost-effective.16 However, despite clinical guidelines, a number of overseas studies report underutilisation of non-pharmacological therapies, inter-professional variation in prescription of non-pharmacological interventions, and use of physical therapies with little evidence of effectiveness.8,17-19 Multidisciplinary care, including physical therapy, increases the likelihood of being prescribed comprehensive therapy.18
In Australia, there are insufficient data about guideline implementation for osteoarthritis. In a baseline assessment of 27 medical records of new patients attending a public hospital’s osteoarthritis hip and knee clinic, there was poor documentation of NSAID risk assessment (1/12), blood pressure (3/12 taking NSAIDs) and emotional state (3/27). Only three of 27 patients reported having previously had exercise program prescription, and none had had nutrition assessment, although the median body mass index was 32.5 kg/m2 (interquartile range, 29.2–37.9 kg/m2) (unpublished data; available from the author). Although documentary inadequacies may have underestimated adherence to recommendations, these findings are in keeping with a recent report.8 Further qualitative data identified gaps in meeting perceived needs for medication management and continuity of care.20
There are multiple system, clinician and patient factors that influence service capacity to effectively implement clinical guidelines.21 In a previous osteoarthritis mapping study, the major barrier we identified was systemic, with poorly integrated osteoarthritis services within, and between, acute and community settings.22
Some patients with chronic conditions do not continue to adhere to therapeutic recommendations after 6 months,23 and osteoarthritis patients are among those with the highest non-adherence rates.24 Long-term adoption of non-pharmacological recommendations is more likely if interventions are perceived to be efficacious and to positively affect outcomes.25 Self-management programs aim to support patient self-management, which has been defined as “active participation by people in their own health care”.2 In Australia, two programs are well defined:
The Arthritis Self-Management Program, a formal, structured group model led by health professionals or trained peers and run over a 6-week period, incorporates educational and behavioural strategies designed to improve patient self-efficacy or the “confidence to do”.26 However, recent evidence suggests that benefits are minimal and limited to psychological endpoints, especially if provided in isolation from physical therapy.27,28
The Flinders Model is a “one-on-one” service model that focuses on supporting behaviour change using goal-setting care plans.29
The comparative reach, applicability and cost-effectiveness of different self-management programs for osteoarthritis is unknown.30
In addition to self-management support, additional chronic disease management components are required to support health care professionals to apply best practice, thereby helping patients manage all aspects of their condition in relation to their daily lives.31
The challenge of addressing behaviour change was highlighted in a systematic review of interventions to enhance medication adherence.32 Although not specific for patients with osteoarthritis, the review reported that interventions that were effective for long-term care were complex and included combinations of interventions such as information, reminders, self-monitoring, reinforcement, counselling, family therapy, psychological therapy, crisis intervention, telephone follow-up and supportive care.32
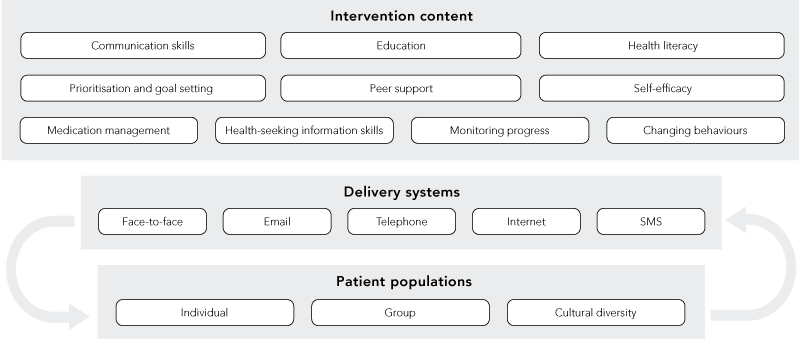
Therefore, an ideal chronic disease management model would include practical service delivery components such as evidence-based clinician and consumer decision aids, health care providers with communication skills,33 and condition monitoring systems. To achieve this, we need to better understand the “black box” of chronic disease management — the elements that form the construct of self-management, how these are tailored to individual need, and how this influences service design and delivery (Box 3). It is difficult to predict how many patients will be incapable of participating, or will choose not to participate in active decision making. However, such patients are likely to be part of the most disenfranchised populations, which are at the greatest health risk.34
We need to use robust research methods to investigate innovative service delivery models such as telephone support35 and coaching programs,36 as an individual with osteoarthritis might prioritise and adopt interventions differently to people with other chronic conditions.
Although chronic disease management taxonomy is not yet standardised, common structural service components have been described. These include an organised system with a culture of quality and safety,37 evidence-based care supported by clinical decision support tools, continuous care supported by clinical information systems, program participation focused at a community level, and supported patient self-management.38 In this primary care model of chronic disease management, the GP provides leadership and works with allied health professionals, pharmacists and medical specialists.38
This model is ideal for osteoarthritis, where acute care intervention is limited to patients with severe disease (for whom joint replacement surgery is indicated). However, although there is high-level evidence of a positive association between chronic disease management models and adherence to best-practice guidelines and patient health outcomes for other chronic conditions, the evidence for osteoarthritis is lacking.39 This may reflect an absence of chronic disease management components, other than self-management support, in reported osteoarthritis service models.39,40
There remain a number of generic and specific barriers to implementation of chronic disease management models for osteoarthritis management in Australia. Clinical information systems, which can support effective integration of decision support for guideline implementation, are well developed in general practice but are poorly integrated with other health care providers.41 Further, we have no national infrastructure comparable to the Scottish Intercollegiate Guidelines Network to support update of knowledge resources. Meeting the information needs of a diverse range of patients with osteoarthritis is also a challenge.
Service delivery is inhibited by workforce constraints in all professions. As a result, role redefinition using innovative service delivery models — such as those led by musculoskeletal coordinators who, using mutually agreed-upon protocols to comprehensively assess patients, coordinate care and provide self-management support — needs to be further investigated.42
Finally, funding models must support chronic care delivery. Integrated acute and community setting chronic disease management models for chronic heart failure and chronic obstructive pulmonary disease, driven by the need to address rising admissions, are supported by jurisdictional funding mechanisms such as the Hospital Admission Risk Program in Victoria.43 Recent Enhanced Primary Care44 funding initiatives to support primary care chronic disease management items through the Medicare Benefits Schedule (item numbers 721–731) provide limited access to allied health professionals, but do not address all the barriers to implementing chronic care service components. Therefore, the degree to which NCDS policy will be effectively implemented for conditions managed primarily in community settings, such as osteoarthritis, is uncertain.
1 Aims of the National Chronic Disease Strategy (NCDS)2
Prevent or delay the onset of chronic disease
Reduce the progression and complications of chronic disease
Maximise wellbeing and quality of life for people living with chronic disease and their families and carers
Reduce avoidable hospitalisation and health care procedures
Implement best practice in prevention, detection, and management of chronic disease
Enhance workforce capacity to meet population demand for chronic disease prevention and care
Adopt a population health approach and reduce health inequalities
Prioritise health promotion and illness prevention
Achieve patient-centred care and optimise self-management
Provide the most effective care
Facilitate coordinated and multidisciplinary care across settings and sectors
Achieve significant and sustainable change
Monitor progress
2 Evidence-based interventions for management of symptomatic osteoarthritis of the hip and/or knee*
Weight reduction for overweight or obese individuals (Grade B)†
Land-based exercise programs (Grade B)
Simple analgesia (paracetamol) (Grade A)
Oral non-steroidal anti-inflammatory drugs‡ (Grade B)
Intra-articular corticosteroid injections (Grade B)
Weak and strong opioid medications‡ (Grade A)
* Adapted from Royal Australian College of General Practitioners guidelines for conservative management of osteoarthritis of the hip and knee.9 † National Health and Medical Research Council (NHMRC) grades of evidence. Grade A = excellent evidence; body of evidence can be trusted to guide practice. Grade B = good evidence; body of evidence can be trusted to guide practice in most situations.10 ‡ These medications should be used cautiously and with appropriate monitoring in view of their known side-effect profile.
- Caroline A Brand1,2,3
- 1 Clinical Epidemiology and Health Service Evaluation Unit, Royal Melbourne Hospital, Melbourne, VIC.
- 2 Centre For Research Excellence in Patient Safety, Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
- 3 Department of Medicine, University of Melbourne, Melbourne, VIC.
The information pertaining to audit data about osteoarthritis patients was supported by a Commonwealth Government peer-reviewed Arthritis and Musculoskeletal Quality Improvement Program Grant. I received reimbursement from the Royal Australian College of General Practitioners for contributing to the development of the osteoarthritis guidelines.9
- 1. Mathers C, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 2006; 3: e442.
- 2. National Health Priority Action Council. National Chronic Disease Strategy. Canberra: Australian Government Department of Health and Ageing, 2006. http://www.health.gov.au/internet/main/publishing.nsf/Content/7E7E9140A3D3A3BCCA257140007AB32B/$File/stratal3.pdf (accessed Sep 2008).
- 3. Woolf A, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003; 81: 646-656.
- 4. March L, Bagga H. Epidemiology of osteoarthritis in Australia. Med J Aust 2004; 180 (5 Suppl): S6-S10. <MJA full text>
- 5. Access Economics. Painful realities: the economic impact of arthritis in Australia in 2007. Sydney: Arthritis Australia, 2007.
- 6. Britt H, Miller GC, Charles J, et al. General practice activity in Australia 2005–06. Canberra: Australian Institute of Health and Welfare, 2007. (AIHW Cat. No. GEP 19.)
- 7. Graves SE, Davidson D, Ingerson L, et al. The Australian Orthopaedic Association National Joint Replacement Registry. Med J Aust 2004; 180 (5 Suppl): S31-S34. <MJA full text>
- 8. DeHaan M, Guzman J, Bayley M, et al. Knee osteoarthritis clinical practice guidelines — how are we doing? J Rheumatol 2007; 34: 2099-2105.
- 9. Royal Australian College of General Practitioners. Hip and knee osteoarthritis: clinical guideline. Melbourne: RACGP, 2008. http://www.racgp.org.au/Content/NavigationMenu/ClinicalResources/RACGPGuidelines/Arthritis/OAguideline.pdf (accessed Sep 2008).
- 10. Coleman K, Norris S, Weston A, et al. NHMRC additional levels of evidence and grades for recommendations for developers of guidelines — pilot program 2005–2007. Canberra: National Health and Medical Research Council, 2005. http://www.nhmrc.gov.au/guidelines/_files/levels_grades05.pdf (accessed Sep 2008).
- 11. National Institute for Health and Clinical Excellence. Osteoarthritis: the care and management of osteoarthritis in adults. London: NICE, 2008. http://www.nice.org.uk/nicemedia/pdf/CG59NICEguideline.pdf (accessed Sep 2008).
- 12. Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003; 62: 1145-1155.
- 13. Zhang W, Doherty M, Arden N, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2005; 64: 669-681.
- 14. Belza B, Topolski T, Kinne S, et al. Does adherence make a difference? Results from a community-based aquatic exercise program. Nurs Res 2002; 51: 285-291.
- 15. Higashi T, Shekelle PG, Adams JL KC, et al. Quality of care is associated with survival in vulnerable older patients. Ann Intern Med 2005; 143: 274-281.
- 16. Andrews G, Simonella L, Lapsley H, et al. Evidence-based medicine is affordable: the cost-effectiveness of current compared with optimal treatment in rheumatoid and osteoarthritis. J Rheumatol 2006; 33: 671-680.
- 17. Ganz DA, Chang JT, Roth CP, et al. Quality of osteoarthritis care for community-dwelling older adults. Arthritis Rheum 2006; 55: 241-247.
- 18. Glazier RH, Badley EM, Wright JG, et al. Patient and provider factors related to comprehensive arthritis care in a community setting in Ontario, Canada. J Rheumatol 2003; 30: 1846-1850.
- 19. Sarzi-Puttini P, Cimmino MA, Scarpa R, et al. Do physicians treat symptomatic osteoarthritis patients properly? Results of the AMICA experience. Semin Arthritis Rheum 2005; 35: 38-42.
- 20. Manias E, Claydon-Platt K, McColl G, et al. Managing complex medication regimens: perspectives of consumers with osteoarthritis and healthcare professionals. Ann Pharmacother 2007; 41: 764-771.
- 21. Greenhalgh T, Robert G, Macfarlane F, et al. Diffusion of innovations in service organizations: systematic review and recommendations. Milbank Q 2004; 82: 581-629.
- 22. Brand C, Cox S. Systems for implementing best practice for a chronic disease: management of osteoarthritis of the hip and knee. Intern Med J 2006; 36: 170-179.
- 23. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005; 353: 487-497.
- 24. DiMatteo M, Haskard K, Williams S. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care 2007; 45: 521-528.
- 25. Damush T, Perkins S, Mikesky A, et al. Motivational factors influencing older adults diagnosed with knee osteoarthritis to join and maintain an exercise program. J Aging Phys Act 2005; 13: 45-60.
- 26. Lorig K, Gonzàlez VM, Laurent DD, et al. Arthritis self-management program variations: three studies. Arthritis Care Res 1998; 11: 448-454.
- 27. Buszewicz M, Rait G, Griffin M, et al. Self management of arthritis in primary care: randomised controlled trial. BMJ 2006; 333: 879.
- 28. Devos-Comby L, Cronan T, Roesch SC. Do exercise and self-management interventions benefit patients with osteoarthritis of the knee? A metaanalytic review. J Rheumatol 2006; 33: 744-756.
- 29. Battersby M, Harvey P, Mills PD, et al. SA HealthPlus: a controlled trial of a statewide application of a generic model of chronic illness care. Milbank Q 2007; 85: 37-67.
- 30. Jordan JE, Osborne RH. Chronic disease self-management education programs: challenges ahead. Med J Aust 2007; 186: 84-87. <MJA full text>
- 31. Glasziou P, Haynes B. The paths from research to improved health outcomes. Evid Based Nurs 2005; 8: 36-38.
- 32. Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008; (2): CD000011. doi: 10.1002/14651858.: CD000011.pub3.
- 33. Greenberg PB, Walker C, Buchbinder R. Optimising communication between consumers and clinicians [editorial]. Med J Aust 2006; 185: 246-247. <MJA full text>
- 34. Wamala S, Merlo J, Bostrom G, et al. Socioeconomic disadvantage and primary non-adherence with medication in Sweden. Int J Qual Health Care 2007; 19:134-140.
- 35. Maisiak R, Austin J, Heck L. Health outcomes of two telephone interventions for patients with rheumatoid arthritis or osteoarthritis. Arthritis Rheum 1996; 39: 1391-1399.
- 36. Edelman D, Oddone EZ, Liebowitz RS, et al. A multidimensional integrative medicine intervention to improve cardiovascular risk. J Gen Intern Med 2006; 21: 728-734.
- 37. Kohn LT, Corrigan JM, Donaldson MS, editors. To err is human: building a safer health system. Executive summary. Washington, DC: National Academy Press, 1999.
- 38. Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. JAMA 2002; 288: 1909-1914.
- 39. Zwar N, Harris M, Griffiths R, et al. A systematic review of chronic disease management. Canberra: Australian Primary Health Care Research Institute, 2006. http://www.anu.edu.au/aphcri/Domain/ChronicDiseaseMgmt/Approved_25_Zwar.pdf (accessed Apr 2007).
- 40. Chodosh J, Morton SC, Mojica W, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med 2005; 143: 427-438.
- 41. Kawamoto K, Houlihan C, Balas E, et al. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005; 330: 765.
- 42. Oldmeadow LB, Bedi HS, Burch HT, et al. Experienced physiotherapists as gatekeepers to hospital orthopaedic outpatient care. Med J Aust 2007; 186: 625-628. <MJA full text>
- 43. Emergency Demand Coordination Group, Victorian Government Department of Human Services. Hospital Admission Risk Program (HARP). Background paper. Melbourne: DHS, 2002. http://www.health.vic.gov.au/harp-cdm/harpbgpap.pdf (accessed Sep 2008).
- 44. Australian Government Department of Health and Ageing. Medicare Benefits Schedule — note A.33. EPC care planning (Items 721 to 731). Chronic disease management items (721-731). http://www9.health.gov.au/mbs/fullDisplay.cfm?type=note&q=A.33 (accessed Sep 2008).





Abstract
Osteoarthritis of the hip and knee is an increasingly common condition that is managed principally with lifestyle behaviour changes. Osteoarthritis management can be complex, as it typically affects older patients with multiple comorbidities.
There is evidence that opportunities exist to improve uptake of evidence-based recommendations for care, especially for non-pharmacological interventions.
The National Chronic Disease Strategy (NCDS) defines key components of programs designed to meet the needs of people with chronic conditions; one component is patient self-management.
NCDS principles have been effectively integrated into chronic disease management programs for other conditions, but there is limited evidence of effectiveness for osteoarthritis programs.
A comprehensive osteoarthritis management model that reflects NCDS policy is needed.
Barriers to implementing such a model include poor integration of decision support, a lack of national infrastructure, workforce constraints and limited funding.