The World Health Organization (WHO) has noted that testing for the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) continues to support global efforts to reduce the morbidity and mortality associated with coronavirus disease 2019 (COVID‐19) by facilitating timely care and treatment and reducing viral transmission.1 Reverse transcription polymerase chain reaction (RT‐PCR) testing is the reference standard for detecting SARS‐CoV‐2 infections.2 However, as RT‐PCR testing in Australia now requires a referral from a general practitioner or nurse practitioner,3 self‐testing using rapid antigen tests (RATs) has become the main method for identifying SARS‐CoV‐2 infections.4 RATs are readily available from pharmacies, retail outlets, and online suppliers.
In Australia, the Therapeutic Goods Administration (TGA) first approved RATs for self‐testing in November 2021.5 By 1 September 2022, 53 RATs had been approved, each with an entry in the Australian Register of Therapeutic Goods,6 classified by the TGA according to the estimated sensitivity reported by the manufacturer: “acceptable sensitivity” (greater than 80%), “high sensitivity” (greater than 90%), and “very high sensitivity” (greater than 95%).5 However, sensitivity estimates provided by manufacturers may not reflect performance when test kits are used for self‐testing at home.2
For this review, we systematically collated and appraised published evaluations, based upon confirmatory RT‐PCR testing for SARS‐CoV‐2 infections, of the diagnostic accuracy of COVID‐19 RATs approved by the TGA for self‐testing by ambulatory people. We then compared these diagnostic accuracy estimates with manufacturer estimates published on the TGA website.
Methods
Information sources and search strategy
We searched for publications to 1 September 2022 in the Cochrane COVID‐19 Study Register7 and the WHO COVID‐19 research database.8 The two databases are living data repositories, regularly updated by searches of MEDLINE, EMBASE, the bioRxiv and medRxiv preprint servers, and several other databases9,10 using specific search strategies developed by information science specialists.7,8 We report our review according to the PRISMA 2020 guidelines.11
Publication eligibility criteria
To identify potentially relevant records in the two COVID‐19 research repositories, we searched for the following terms: COVID‐19 testing, SARS‐CoV‐2 testing, rapid antigen test*, self‐test*, sensitivity and specificity, diagnostic accuracy, diagnostic performance, and the names of each of the 53 TGA‐approved RATs.
We included publications in any language that described cross‐sectional, case–control, or cohort studies in which the participants were ambulatory people in the community or health care workers in hospitals with suspected SARS‐CoV‐2 infections, and the results of testing self‐collected biological samples with a TGA‐approved COVID‐19 RAT (index test) were compared with those of RT‐PCR testing (reference standard) for SARS‐CoV‐2 infection (target condition).
We excluded publications that reported retrospective studies, and those in which participant selection was not clearly described, the index test was not performed at the time of sample collection, the RAT samples were not collected by the tested person themselves, the sample type or collection method did not comply with the manufacturer's instructions, or the diagnostic accuracy data were not reported or were not adequate for sensitivity and specificity calculations. We also excluded reviews, opinion articles, editorials, and letters that did not include original data, and articles for which the full text was not available.
Study selection and screening
All records identified in the database searches were collated and uploaded to Covidence (Veritas Health Innovation) and duplicates removed. One reviewer (author YL) screened titles and abstracts according to our eligibility criteria. The full text of potentially relevant records was then assessed by one reviewer (YL) according to the eligibility criteria and checked by a second reviewer (KB, EM, or DA); discrepancies were resolved by discussion by the full team.
We downloaded from the TGA database5 the user instructions and clinical data supplied by the manufacturers for the COVID‐19 RATs used in the studies that satisfied our inclusion criteria.
Data extraction
We used a standardised form for extracting information on the characteristics and outcomes of the included studies: author, publication year, country, study design, setting, participant number and characteristics, participant symptoms, RAT brand, test sample type, supervision of sample collection, number of RT‐PCR‐positive participants, reported sensitivity and specificity, and numbers of true positive, false positive, true negative, and false negative test results. Data extraction was undertaken by one reviewer (KB, YL, EM, or DA) and checked by a second (KB, YL, EM, or DA).
Assessment of risk of bias
One reviewer (KB, YL, EM, or DA) assessed the risk of bias (domains: participant characteristics, index test, reference test, flow and timing of tests) for each study using the QUADAS‐2 tool;12 a second reviewer checked each assessment. Disagreements were resolved by consensus.
Analysis
The principal diagnostic accuracy measure for this study was sensitivity per person. We calculated sensitivity and specificity for each study from the reported numbers of true positive, false positive, false negative, and true negative test results to check the sensitivity and specificity reported by the study authors. We then compared the publication values with those reported by the respective manufacturers and published on the TGA website.
We plotted paired sensitivity and specificity estimates for each study in a forest plot using the R package DTAplots;13 the PRISMA flowchart was generated with the PRISMA2020 Shiny app;14 and the risk of bias assessment figures were constructed with the robvis Shiny app.15
Ethics approval
For this negligible risk research study we analysed publicly available, non‐identifiable aggregated data, and our investigation was therefore exempt from formal ethics review. The review protocol was not registered.
Results
A total of 1842 unique potentially relevant records were identified by the Cochrane and WHO database searches. The full text of 296 articles was screened, of which 284 were excluded (Supporting Information, figure 1). Twelve studies were included in our review, all in English: ten peer‐reviewed journal articles17,18,20,21,22,23,24,25,26,27 and two preprints16,19 (Box 1).
The twelve included studies reported eighteen evaluations of RATs in a total of 18 430 participants. Tests by eight manufacturers of TGA‐approved COVID‐19 RATs were evaluated: All Test,20 Roche,16,17,20 Flowflex,21,22 MP Biomedicals,21,22 Clungene,19 Panbio18,23,24,26,27 (two:23,27 BinaxNOW, the United States name for the Panbio test), V‐Chek,25 and Whistling.25 No eligible study was identified for 45 of the 53 TGA‐approved tests. None of the included studies were funded by test manufacturers. The authors of one study declared potential financial conflicts of interest,24 while the authors of eleven studies declared no potential conflicts of interest. Five studies were undertaken in the Netherlands,16,17,20,21,22 two each in Germany19,24 and the United States,23,27 and one each in Denmark,18 Belgium,25 and Canada26 (Box 1).
Sample collection was unsupervised in twelve studies;16,17,18,19,20,21,22,23,24,25,26,27 in six studies sample collection supervised by health care workers or researchers.23,24,25,26,27 The sample sizes for the eighteen evaluations ranged from 50 to 2819 participants, the mean or median age from 31 to 41 years, and the proportion of women from 50.5% to 83%. In one study, the participants did not have symptoms suggesting SARS‐CoV‐2 infection,22 and in two studies participants did not report any symptoms;19,26 in the other evaluations, most participants had symptoms consistent with COVID‐19 or had been in close contact with someone with a confirmed SARS‐CoV‐2 infection. In the studies that reported rates of vaccination and prior COVID‐19, they differed according to study date; for example, 94.5% of participants in the January 2021 Danish study had not received a vaccine dose,18 whereas more than 50% of participants in the Dutch study undertaken in early 2022 had received three vaccine doses22 (Box 1).
Risk of bias: published studies
The risk of bias in the participant characteristics domain was low for five studies;16,17,20,21,22 it was high for one study because it included participants known to be SARS‐CoV‐2‐positive,27 and possibly inappropriate exclusions and inclusions led to concerns about this domain for six studies.18,19,23,24,25,26 The risk of bias for the index test was low for all but one study, for which concerns were raised by the possibility that participants were not blinded to their RT‐PCR reference test results at the time of the RAT.18 Risk of bias for the reference test was low for nine studies; for three studies,24,25,26 concerns were related to RATs being conducted under the supervision of health care workers or researchers in hospitals, so that index test results may have been known to the laboratory undertaking the RT‐PCR (ie, reference test operators were not blinded to index test results). The risk of bias for flow and timing of tests was low for ten studies; the risk was high in one study because of the large proportion of invalid test results (excluded from the analysis),25 and for a second study because some index tests were undertaken as long as 72 hours after sample collection for the reference test18 (Supporting Information, figure 2).
Risk of bias: manufacturer‐supplied information
Manufacturers provided no information about participant selection in six studies; for two studies at high risk of bias in this domain, we deemed a case–control design or non‐consecutive recruitment likely, as about half the participants in each study were SARS‐CoV‐2‐positive. For the index test domain, risk of bias was low for the Roche study, and seven manufacturers provided no information about the index test. For the reference standard domain, the risk of bias was high for the Panbio study because the reference standard was a RAT version not intended for self‐use; seven manufacturers provided no information about the reference standard. For the flow and timing domain, risk of bias was low for the Roche study and high for the VChek study (results were reported per sample rather than per person); six manufacturers provided no information for this domain (Supporting Information, figure 3).
Diagnostic accuracy
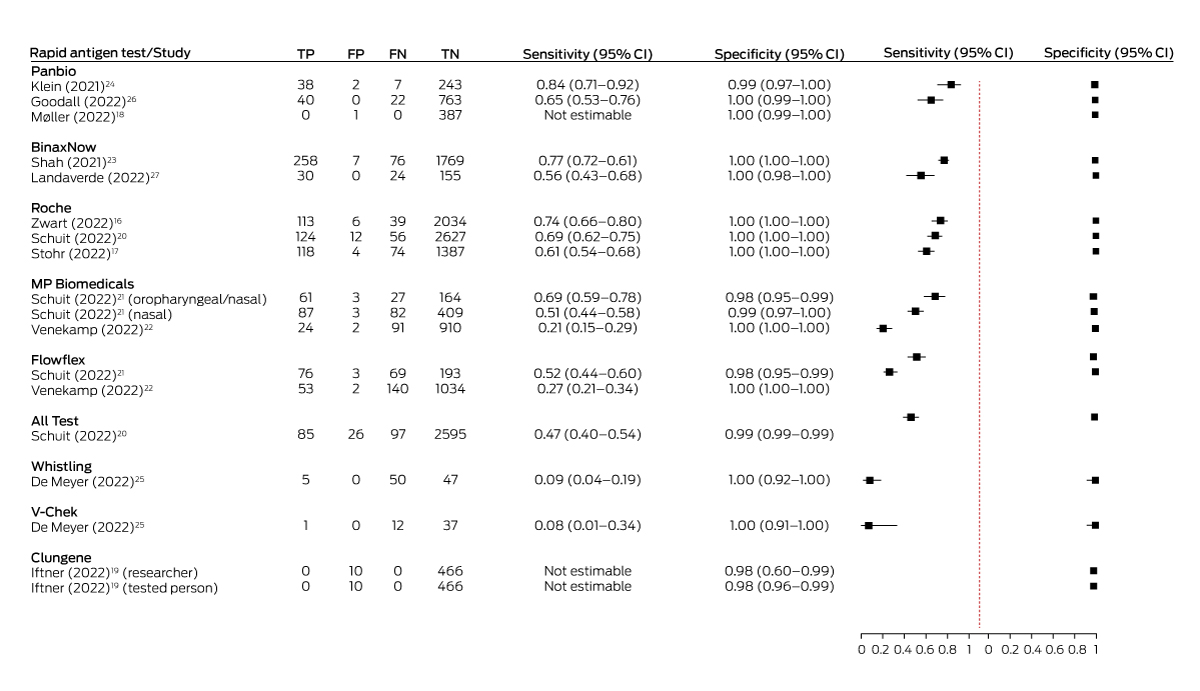
Estimated sensitivity with unsupervised sample collection ranged from 20.9% (MP Biomedicals)22 to 74.3% (Roche),16 and with supervised collection from 7.7% (V‐Chek)25 to 84.4% (Panbio).24 The estimates were between 8.2 and 88 percentage points lower than the sensitivity values reported by the manufacturer and published on the TGA website (Box 2). Our calculations of test sensitivity from data supplied in the published studies concurred with those reported in the studies (Box 3).
Four studies directly compared different TGA‐approved tests, including three in the Dutch public testing system and at low risk of bias across all domains. In two of the Dutch studies, the participants were people with COVID‐19 symptoms: in the first study, the sensitivity of the Roche SD Biosensor nasal self‐test (68.9%) was higher than that of the All Test oral self‐test (46.7%);20 in the second the sensitivity of the MP Biomedicals self‐test with combined oropharyngeal and nasal sampling (69.3%) was higher than that of the MP Biomedicals (51.5%) and Flowflex nasal self‐tests (52.4%).21 The third Dutch study, in which participants did not have COVID‐19 symptoms, the sensitivity of the Flowflex (27.5%) and the MP Biomedicals nasal self‐tests (20.9%) was similarly low.22 The fourth study that compared TGA‐approved tests, in a Belgian university hospital outpatient testing clinic, found that the sensitivity of both the V‐Chek (7.7%) and Whistling saliva tests (9.1%), with supervised sample collection, was very low.25 Sensitivity could not be estimated in the two studies in which no participants were SARS‐CoV‐2‐positive by RT‐PCR.18,19
Estimated specificity was high for all tests (97.9–100%) and within 2.5 percentage points of the values reported by manufacturers (Supporting Information, table).
Discussion
At the time of our literature search (1 September 2022), the TGA had approved 53 COVID‐19 RATs for self‐use, of which eight had been evaluated in the twelve studies included in our review, including one test (Whistling) for which TGA approval has since been revoked. Further, only seven of the twelve studies included in our review (evaluations of six TGA‐approved RATs) were based upon unsupervised sample collection and interpretation. No relevant studies have been undertaken in Australia, despite the extremely high rates of COVID‐19 testing in this country during 2020–22;28 comparative diagnostic accuracy studies in the public testing system could have provided valuable local evidence regarding RATs approved or under consideration by the TGA. The scarcity of robust evidence for the diagnostic accuracy of these tests when used as intended (ie, without supervision by medical personnel) is striking.
Estimated sensitivity varied substantially between tests and studies and were (often substantially) lower than the manufacturer‐reported values on the TGA website. The consequence is that the risk of false negative results is high when these tests are used for self‐testing at home, a problem unlikely to be appreciated by people who inform themselves on the TGA website about test performance. Decisions based on false negative results could hamper control of SARS‐CoV‐2 transmission, as infected people may decide self‐isolation is unnecessary, thereby unintentionally exposing others to the virus, including older people and others at high risk of morbidity and mortality. Infected people with false negative results are also less likely to seek RT‐PCR testing, especially as it now requires referral by a medical or nurse practitioner to a private pathology clinic.3 RT‐PCR tests can detect infections in their early stages, when antiviral treatments are most effective; not undergoing RT‐PCR testing could increase the risk that some people at greater risk of adverse outcomes forgo the possible benefits of early antiviral treatment.
It is reassuring that all eight studies reported high estimated test specificity (at least 97.9%); that is, the likelihood of false positive results is generally low and a positive result usually indicates a genuine infection, especially as tests are now generally motivated by suspicion of COVID‐19 (higher pre‐test probability).29
Our findings are similar to those of earlier systematic reviews, which found that the sensitivity of RATs varied substantially but that their specificity was consistently high.2,30 The 2022 Cochrane review identified two studies that compared the effect of who interpreted RAT results on estimated sensitivity; each found that it was lower when interpreted by participants rather than by a medical practitioner.2 Other factors that can vary substantially between studies, including study design, participant characteristics, and test setting and conduct, can also influence estimated sensitivity. Consequently, indirect (between‐study) comparisons of test performance are difficult to interpret.2,30 However, it is not clear on the TGA website that the sensitivity values listed are derived from different studies and should not be directly compared. It might be assumed that a “high sensitivity” test is more accurate than one with “acceptable sensitivity”. However, a Dutch within‐study comparison found that the Roche nasal self‐test was more sensitive than the All Test oral self‐test,20 for example, but the information on the TGA website could lead to the opposite conclusion (Roche: “acceptable sensitivity”; All Test: “high sensitivity”5).
Limitations
Our review was based on searches in the comprehensive WHO and Cochrane COVID‐19 living data repositories, which employ robust search strategies to identify relevant studies.9,10 Two reviewers were involved in each of the full‐text screening, data extraction, and risk of bias steps of the analysis. However, the identified studies assessed only eight TGA‐approved RATs, and we cannot comment on the diagnostic accuracy of other tests registered with the TGA. Since the time of our searches, the TGA has approved further tests and removed others from the self‐test list (78 TGA‐approved self‐tests were listed on 31 July 2023). Diagnostic accuracy studies for some newer tests may have been published after our search, or TGA approval may have been granted after our search. Risk of bias assessment for manufacturer‐reported studies was limited by the degree of information provided. Finally, the protocol for our systematic review was not registered, and the initial screening of titles and abstracts was undertaken by a single reviewer.
Conclusion
Australia now relies on people isolating themselves and taking other precautionary measures for preventing and controlling COVID‐19, and self‐testing for infection plays a pivotal role in these decisions. However, evidence regarding the diagnostic accuracy of RATS when used as intended (unsupervised sample collection and interpretation) is limited. The substantial difference in sensitivity estimates between those supplied by manufacturers (and used to classify tests on the TGA website) and those from independent published studies is concerning. We hope that our findings will increase community awareness of the high risk of false negative results when using RATs for self‐testing and encourage people to not rule out infection solely on the basis of a RAT result if COVID‐19 is suspected. To improve the transparency of the evidence on its website, the TGA could require manufacturers to report their clinical studies according to the STARD guideline,31 facilitating independent risk of bias assessment with the QUADAS‐2 tool.12 It should also be made clear that the test sensitivity values listed on the TGA website are derived from different studies and should not be directly compared. Finally, we need better designed diagnostic accuracy studies of SARS‐CoV‐2 rapid antigen self‐tests.32
Box 1 – Characteristics of studies of Australian Therapeutic Goods Authority‐approved COVID‐19 rapid antigen self‐tests included in our systematic analysis
|
Author (publication year), country |
Study design and setting |
Participant characteristics and symptoms* |
Rapid antigen test |
||||||||||||
|
|
|||||||||||||||
|
Unsupervised sample collection |
|||||||||||||||
|
Zwart (2022),16 Netherlands |
|
|
Roche SARS‐CoV‐2 Rapid Antigen Test (oropharyngeal and nasal) |
||||||||||||
|
Stohr (2022),17 Netherlands |
|
|
Roche SARS‐CoV‐2 Antigen Self‐Test (nasal) |
||||||||||||
|
Møller (2022),18 Denmark |
|
|
Panbio COVID‐19 Antigen Rapid Test Device (nasal) |
||||||||||||
|
Iftner (2022),19 Germany |
|
|
Clungene COVID‐19 Antigen Rapid Test (nasal, researcher interpretation) |
||||||||||||
|
Clungene COVID‐19 Antigen Rapid Test (nasal; participant interpretation) |
|||||||||||||||
|
Schuit (2022),20 Netherlands |
|
|
All Test COVID‐19 Antigen Rapid Test (oral fluid) |
||||||||||||
|
Roche SARS‐CoV‐2 Antigen Self‐Test (nasal) |
|||||||||||||||
|
Schuit (2022),21 Netherlands |
|
|
Flowflex SARS‐CoV‐2 Antigen Rapid Test (nasal) |
||||||||||||
|
MP Biomedicals Rapid SARS‐COV‐2 Antigen Test Card (nasal) |
|||||||||||||||
|
MP Biomedicals Rapid SARS‐COV‐2 Antigen Test Card (oropharyngeal and nasal) |
|||||||||||||||
|
Venekamp (2023),22 Netherlands |
|
|
Flowflex SARS‐CoV‐2 Antigen Rapid Test (nasal) |
||||||||||||
|
MP Biomedicals Rapid SARS‐COV‐2 Antigen Test Card (nasal) |
|||||||||||||||
|
Supervised sample collection |
|||||||||||||||
|
Shah (2021),23 USA |
|
|
BinaxNOW COVID‐19 Antigen Card Self‐Test† (nasal) |
||||||||||||
|
Klein (2021),24 Germany |
|
|
Panbio COVID‐19 Antigen Rapid Test Device (nasopharyngeal) |
||||||||||||
|
DeMeyer (2022),25 Belgium |
|
|
V‐Chek COVID‐19 Antigen Saliva Test (oral) |
||||||||||||
|
Whistling test 2019‐nCoV Saliva Ag Easy Test (oral) |
|||||||||||||||
|
Goodall (2022),26 Canada |
|
|
Panbio COVID‐19 Antigen Rapid Test Device (nasal) |
||||||||||||
|
Landaverde (2022),27 USA |
|
|
BinaxNOW COVID‐19 Antigen Card Self‐Test† (nasal) |
||||||||||||
|
|
|||||||||||||||
|
COVID‐19 = coronavirus disease 2019; PCR = polymerase chain reaction; SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus; SD = standard deviation; USA = United States of America. * Characteristics and symptoms for Schuit20 refer to All Test evaluation (similar for Roche evaluation); for Venekemp22 to Flowflex evaluation (similar for MP Biomedicals evaluation); for Schuit21 to Flowflex evaluation (similar for MP Biomedicals evaluations) and for all participants, including those undertaking confirmatory testing after prior positive self‐test (characteristics of non‐confirmatory testers not provided); for Møller18 to the overall study, which included other tests not approved by the TGA (characteristics for the Panbio evaluation not provided). Characteristics for De Meyer25 refer to V‐Chek evaluation (similar for Whistling evaluation). † BinaxNOW COVID‐19 Antigen Card Self‐Test, the Abbott test marketed in the USA, is identical to the Panbio COVID‐19 Antigen Rapid Test Device. |
|||||||||||||||
Box 2 – Therapeutic Goods Authority (TGA)‐approved SARS‐CoV‐2 rapid antigen tests for self‐testing: sensitivity as reported in published studies and by test manufacturers
|
Author (publication year), country |
Rapid antigen test (RAT) |
Sample size* |
RT‐PCR‐positive |
Sensitivity reported by study |
Sensitivity reported by manufacturer |
Difference (percentage points) |
|||||||||
|
|
|||||||||||||||
|
Unsupervised sample collection |
|||||||||||||||
|
Zwart (2022),16 Netherlands |
Roche SARS‐CoV‐2 Rapid Antigen Test (oropharyngeal and nasal) |
2192 |
152 |
74.3% |
82.5% |
8.2 |
|||||||||
|
Stohr (2022),17 Netherlands |
Roche SARS‐CoV‐2 Antigen Self‐Test (nasal) |
1583 |
192 |
61.5% |
82.5% |
21.0 |
|||||||||
|
Møller (2022),18 Denmark |
Panbio COVID‐19 Antigen Rapid Test Device (nasal) |
388 |
0 |
NA |
95.2% |
NA |
|||||||||
|
Iftner (2022),19 Germany |
Clungene COVID‐19 Antigen Rapid Test (nasal, researcher interpretation) |
478 |
0 |
NA |
95.1% |
NA |
|||||||||
|
Clungene COVID‐19 Antigen Rapid Test (nasal, participant interpretation) |
476 |
0 |
NA |
95.1% |
NA |
||||||||||
|
Schuit (2022),20 Netherlands |
All Test COVID‐19 Antigen Rapid Test (oral fluid) |
2803 |
182 |
46.7% |
90.1% |
43.4 |
|||||||||
|
Roche SARS‐CoV‐2 Antigen Self‐Test (nasal) |
2819 |
180 |
68.9% |
82.5% |
13.6 |
||||||||||
|
Schuit (2022),21 Netherlands |
Flowflex SARS‐CoV‐2 Antigen Rapid Test (nasal) |
341 |
145 |
52.4% |
97.1% |
44.7 |
|||||||||
|
MP Biomedicals Rapid SARS‐COV‐2 Antigen Test Card (nasal) |
581 |
169 |
51.5% |
98.2% |
46.7 |
||||||||||
|
MP Biomedicals Rapid SARS‐COV‐2 Antigen Test Card (oropharyngeal and nasal) |
255 |
88 |
69.3% |
98.2% |
28.9 |
||||||||||
|
Venekamp (2023),22 Netherlands |
Flowflex SARS‐CoV‐2 Antigen Rapid Test (nasal) |
1229 |
193 |
27.5% |
97.1% |
69.6 |
|||||||||
|
MP Biomedicals Rapid SARS‐COV‐2 Antigen Test Card (nasal) |
1027 |
115 |
20.9% |
82.5% |
61.6 |
||||||||||
|
Supervised sample collection |
|||||||||||||||
|
Shah (2021),23 USA |
BinaxNOW COVID‐19 Antigen Card Self‐Test (nasal) |
2110 |
334 |
77.2% |
95.2%† |
18.0 |
|||||||||
|
Klein (2021),24 Germany |
Panbio COVID‐19 Antigen Rapid Test Device (nasopharyngeal) |
290 |
45 |
84.4% |
95.2% |
10.8 |
|||||||||
|
DeMeyer (2022),25 Belgium |
V‐Chek COVID‐19 Antigen Saliva Test (oral) |
50 |
13 |
7.7% |
95.7% |
88.0 |
|||||||||
|
Whistling test 2019‐nCoV Saliva Ag Easy Test (oral) |
102 |
55 |
9.1% |
93.9% |
84.8 |
||||||||||
|
Goodall (2022),26 Canada |
Panbio COVID‐19 Antigen Rapid Test Device (nasal) |
825 |
62 |
64.5% |
95.2% |
30.7 |
|||||||||
|
Landaverde (2022),27 USA |
BinaxNOW COVID‐19 Antigen Card Self‐Test‡ (nasal) |
209 |
54 |
55.6% |
95.2%† |
39.6 |
|||||||||
|
|
|||||||||||||||
|
COVID‐19 = coronavirus disease 2019; NA = not applicable; RT‐PCR = reverse transcription–polymerase chain reaction SARS‐CoV‐2 = severe acute respiratory syndrome coronavirus; USA = United States of America. * Does not include participants for whom RAT or RT‐PCR results were unclear or inconclusive. † Manufacturer‐reported sensitivity is for Panbio COVID‐19 Antigen Rapid Test Device. |
|||||||||||||||
Received 20 December 2022, accepted 9 August 2023
- Katy JL Bell1
- Yuyang Li1
- Ellie Medcalf1
- Deonna Ackermann1
- School of Public Health, the University of Sydney, Sydney, NSW
Open access:
Open access publishing facilitated by The University of Sydney, as part of the Wiley – The University of Sydney agreement via the Council of Australian University Librarians.
Katy Bell is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (1174523), Ellie Medcalf by an NHMRC Postgraduate Scholarship (2022279) and a PhD Scholarship from Sydney Cancer Partners (with funding from Cancer Institute NSW: 2021/CBG0002), and Deonna Ackermann by an NHMRC Postgraduate Scholarship (2014163). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
No relevant disclosures.
- 1. World Health Organization. WHO policy brief: COVID‐19 testing, (WHO/2019‐nCoV/Policy_Brief/Testing/2022.1). https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐Policy_Brief‐Testing‐2022.1 (viewed Sept 2023).
- 2. Dinnes J, Sharma P, Berhane S, et al; Cochrane COVID‐19 Diagnostic Test Accuracy Group. Rapid, point‐of‐care antigen tests for diagnosis of SARS‐CoV‐2 infection. Cochrane Database Syst Rev 2022; CD013705.
- 3. Australian Department of Health and Aged Care. Testing for COVID‐19. Updated 7 June 2023. https://www.health.gov.au/topics/covid‐19/testing (viewed Sept 2023).
- 4. Meumann EM, Robson JMB. Testing for COVID‐19: a 2023 update. Australian Prescriber 2023; 46: 13‐17.
- 5. Therapeutic Goods Administration (Australian Department of Health and Aged Care). COVID‐19 rapid antigen self‐tests that are approved in Australia. Updated 11 Sept 2023. https://www.tga.gov.au/products/covid‐19/covid‐19‐tests/covid‐19‐rapid‐antigen‐self‐tests‐home‐use/covid‐19‐rapid‐antigen‐self‐tests‐are‐approved‐australia (viewed Sept 2023).
- 6. Therapeutic Goods Administration (Australian Department of Health and Aged Care). Australian Register of Therapeutic Goods (ARTG). Updated 6 Jan 2022. https://www.tga.gov.au/products/australian‐register‐therapeutic‐goods‐artg (viewed Sept 2023).
- 7. Cochrane Community. About COVID‐19 Study Register. Updated June 2022. https://community.cochrane.org/about‐covid‐19‐study‐register (viewed Sept 2023).
- 8. World Health Organization. WHO COVID‐19 Research Database: user guide and information. 29 June 2022. https://www.who.int/publications/m/item/quick‐search‐guide‐who‐covid‐19‐database (viewed Sept 2023).
- 9. Butcher R, Sampson M, Couban RJ, et al. The currency and completeness of specialized databases of COVID‐19 publications. J Clin Epidemiol 2022; 147: 52‐59.
- 10. Pierre O, Riveros C, Charpy S, Boutron I. Secondary electronic sources demonstrated very good sensitivity for identifying studies evaluating interventions for COVID‐19. J Clin Epidemiol 2022; 141: 46‐53.
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71.
- 12. Whiting PF, Rutjes AW, Westwood ME, et al; QUADAS‐2 Group. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529‐536.
- 13. Doebler P, Holling H, Sousa‐Pinto B. Meta‐analysis of diagnostic accuracy with mada. The Comprehensive R Archive Network. https://cran.r‐project.org/web/packages/mada/vignettes/mada.pdf (viewed Sept 2023).
- 14. Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and Shiny app for producing PRISMA 2020‐compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst Rev 2022; 18: e1230.
- 15. McGuinness LA, Higgins JPT. Risk‐of‐bias VISualization (robvis): an R package and Shiny web app for visualizing risk‐of‐bias assessments. Res Synth Methods 2021; 12: 55‐61.
- 16. Zwart VF, van der Moeren, Stohr JJJM, et al. Performance of various lateral flow SARS‐CoV‐2 antigen self testing methods in healthcare workers: a multicenter study [preprint]. medRxiv 2022.01.28.22269783; 29 Jan 2022. https://doi.org/10.1101/2022.01.28.22269783 (viewed Sept 2023).
- 17. Stohr JJJM, Zwart VF, Goderski G, et al. Self‐testing for the detection of SARS‐CoV‐2 infection with rapid antigen tests for people with suspected COVID‐19 in the community. Clin Microbiol Infect 2022; 28: 695‐700.
- 18. Møller IJB, Utke AR, Rysgaard UK, et al. Diagnostic performance, user acceptability, and safety of unsupervised SARS‐CoV‐2 rapid antigen‐detecting tests performed at home. Int J Infect Dis 2022; 116: 358‐364.
- 19. Iftner T, Iftner A, Pohle D, Martus P. Evaluation of the specificity and accuracy of SARS‐CoV‐2 rapid antigen self‐tests compared to RT‐PCR from 1015 asymptomatic volunteers. medRxiv 2022.02.11.22270873; 13 Feb 2022. https://doi.org/10.1101/2022.02.11.22270873 (viewed Sept 2023).
- 20. Schuit E, Venekamp RP, Veldhuijzen IK, et al. Head‐to‐head comparison of the accuracy of saliva and nasal rapid antigen SARS‐CoV‐2 self‐testing: cross‐sectional study. BMC Med 2022; 20: 406.
- 21. Schuit E, Venekamp RP, Hooft L, et al. Diagnostic accuracy of covid‐19 rapid antigen tests with unsupervised self‐sampling in people with symptoms in the omicron period: cross sectional study. BMJ 2022; 378: e071215.
- 22. Venekamp RP, Schuit E, Hooft L, et al. Diagnostic accuracy of SARS‐CoV‐2 rapid antigen self‐tests in asymptomatic individuals in the omicron period: a cross‐sectional study. Clin Microbiol Infect 2023; 29: 391.e1‐391.e7.
- 23. Shah MM, Salvatore PP, Ford L, et al. Performance of repeat BinaxNOW severe acute respiratory syndrome coronavirus 2 antigen testing in a community setting, Wisconsin, November 2020 – December 2020. Clin Infect Dis 2021; 73 (Suppl 1): S54‐S57.
- 24. Klein JAF, Krüger LJ, Tobian F, et al. Head‐to‐head performance comparison of self‐collected nasal versus professional‐collected nasopharyngeal swab for a WHO‐listed SARS‐CoV‐2 antigen‐detecting rapid diagnostic test. Med Microbiol Immunol 2021; 210: 181‐186.
- 25. De Meyer J, Goris H, Mortelé O, et al. Evaluation of saliva as a matrix for RT‐PCR analysis and two rapid antigen tests for the detection of SARS‐CoV‐2. Viruses 2022; 14: 1931.
- 26. Goodall BL, LeBlanc JJ, Hatchette TF, et al. Investigating the sensitivity of nasal or throat swabs: combination of both swabs increases the sensitivity of SARS‐CoV‐2 rapid antigen tests. Microbiol Spectr 2022; 10: e0021722.
- 27. Landaverde L, Turcinovic J, Doucette‐Stamm L, et al. Comparison of BinaxNOW and SARS‐CoV‐2 qRT‐PCR detection of the omicron variant from matched anterior nares swabs. Microbiol Spectr 2022; 10: e0130722.
- 28. Australian Bureau of Statistics. Household Impacts of COVID‐19 Survey. 16 Mar 2022. https://www.abs.gov.au/statistics/people/people‐and‐communities/household‐impacts‐covid‐19‐survey/feb‐2022 (viewed Oct 2023).
- 29. Bell KJ, Stanaway FF, Irwig LM, et al. How to use imperfect tests for COVID‐19 (SARS‐CoV‐2) to make clinical decisions. Med J Aust 2021; 214: 69‐73. https://www.mja.com.au/journal/2021/214/2/how‐use‐imperfect‐tests‐covid‐19‐sars‐cov‐2‐make‐clinical‐decisions
- 30. Brümmer LE, Katzenschlager S, McGrath S, et al. Accuracy of rapid point‐of‐care antigen‐based diagnostics for SARS‐CoV‐2: an updated systematic review and meta‐analysis with meta‐regression analyzing influencing factors. PLoS Med 2022; 19: e1004011.
- 31. Bossuyt PM, Reitsma JB, Bruns DE, et al; STARD Group. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015; 351: h5527.
- 32. Doust JA, Bell KJL, Leeflang MMG, et al. Guidance for the design and reporting of studies evaluating the clinical performance of tests for present or past SARS‐CoV‐2 infection. BMJ 2021; 372: n568.






Abstract
Objectives: To review evaluations of the diagnostic accuracy of coronavirus disease 2019 (COVID‐19) rapid antigen tests (RATs) approved by the Therapeutic Goods Administration (TGA) for self‐testing by ambulatory people in Australia; to compare these estimates with values reported by test manufacturers.
Study design: Systematic review of publications in any language that reported cross‐sectional, case–control, or cohort studies in which the participants were ambulatory people in the community or health care workers in hospitals in whom severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection was suspected, and the results of testing self‐collected biological samples with a TGA‐approved COVID‐19 RAT were compared with those of reverse transcription–polymerase chain reaction (RT‐PCR) testing for SARS‐CoV‐2. Estimates of diagnostic accuracy (sensitivity, specificity) were checked and compared with manufacturer estimates published on the TGA website.
Data sources: Publications (to 1 September 2022) identified in the Cochrane COVID‐19 Study Register and the World Health Organization COVID‐19 research database. Information on manufacturer diagnostic accuracy evaluations was obtained from the TGA website.
Data synthesis: Twelve publications that reported a total of eighteen evaluations of eight RATs approved by the TGA for self‐testing (manufacturers: All Test, Roche, Flowflex, MP Biomedicals, Clungene, Panbio, V‐Chek, Whistling) were identified. Five studies were undertaken in the Netherlands, two each in Germany and the United States, and one each in Denmark, Belgium, and Canada; test sample collection was unsupervised in twelve studies, and supervised by health care workers or researchers in six. Estimated sensitivity with unsupervised sample collection ranged from 20.9% (MP Biomedicals) to 74.3% (Roche), and with supervised collection from 7.7% (V‐Chek) to 84.4% (Panbio); the estimates were between 8.2 and 88 percentage points lower than the values reported by the manufacturers. Test specificity was high for all RATs (97.9–100%).
Conclusions: The risk of false negative results when using COVID‐19 RATs for self‐testing may be considerably higher than apparent in manufacturer reports on the TGA website, with implications for the reliability of these tests for ruling out infection.