The known: Universal screening of all Australians born before 2000 has been discussed as a solution to low rates of chronic hepatitis B diagnosis.
The new: Universal hepatitis B virus screening can be cost‐effective in Australia, but requires high levels of engagement by people with hepatitis B with clinical management. Other factors that influence the cost‐effectiveness of universal testing are the its cost and the test positivity rate.
The implications: To be cost‐effective, universal screening must be based on low cost testing and an effective system for ensuring that people diagnosed receive appropriate clinical management.
Chronic hepatitis B, caused by the hepatitis B virus (HBV), can be asymptomatic for decades before life‐threatening complications develop, including cirrhosis and hepatocellular carcinoma.1,2 Highly effective antiviral therapy reduces the risks of HBV‐related complications and death, but in 2019 only 30 million of 296 million people with chronic hepatitis B had been diagnosed.2
In 2020, an estimated 222 559 people in Australia (0.87%) were living with chronic hepatitis B, including 60 079 unaware of their infection (27%).3 Despite increases in the proportions of people diagnosed and receiving clinical care since 2011,3 modelling studies4,5 suggest that testing rates in Australia are inadequate for meeting World Health Organization hepatitis B elimination targets for 2030, including the diagnosis of 90% of people with HBV infections.2,6 The national hepatitis B testing policy7 recommends HBV testing for people with evidence of liver disease or risk factors for HBV infection, and mandatory testing of blood and tissue donors and people working in health care. Pregnant women are the only group routinely screened for HBV infection in Australia. Recommended testing for hepatitis B (for the surface antigen [HBsAg] or for antibodies to the surface and core antigens [anti‐HBsAg, anti‐HBcAg]) is subsidised by Medicare (Medical Benefits Schedule [MBS] item numbers 69475 and 69481; but can be billed under other item numbers in specific situations, such as pregnancy).
However, symptom‐based testing captures only a small proportion of people with HBV infections, usually those with acute or active hepatitis or symptoms of advanced liver disease. Risk‐based testing is often inadequate in general practice;8 an analysis of data for almost 1.7 million adults who attended 456 Victorian general practices found that fewer than 30% of those with risk factors for hepatitis B or C testing were tested,3 highlighting the gap between testing practice and policy.6,9 Challenges for primary care providers regarding risk‐based HBV testing include inadequate knowledge about at‐risk groups, lack of information regarding individual patient risk, and fear of being accused of over‐servicing.10,11
A universal screening strategy could increase HBV testing and diagnosis rates, especially if it targeted people born before 2000; infant HBV vaccination coverage has been high in Australia and overseas since 2000.8,12 General practitioners could opportunistically test all patients born before 2000 without records of previous HBV testing. This approach would simplify risk assessment, reduce barriers to primary care providers offering testing, and overcome the fear of stigmatisation among people in at‐risk groups.
Two critical factors for a universal screening strategy are its cost‐effectiveness and affordability, particularly in light of the low HBV prevalence in Australia, the scale of testing required, and the cost of establishing a new screening program. Further, pathways for pre‐test counselling and post‐test referral to appropriate care would be needed.
As no economic evaluation of a universal HBV screening strategy for Australia has been published, we assessed the impact, cost, and cost‐effectiveness of two options in a Markov model, compared with current HBV testing practice: universal HBV screening of people born before 2000, with the aim of reaching the WHO 90% diagnosis target by 2030; and combining universal screening with more people receiving appropriate care, with the aim of ensuring that 50% of people with chronic hepatitis B are receiving appropriate clinical care.
Methods
We explored the impact of a novel testing intervention in a specifically adapted Markov model of hepatitis B disease transition in Australia5 (Supporting Information, figure 1). The model was initialised with 222 559 people with chronic hepatitis B in 2020;3 baseline epidemiological characteristics of the model cohort (distribution by age, sex, ethnic background, disease state, and care cascade) are summarised in the Supporting Information, tables 1 to 3.
We based annual probabilities of disease progression (untreated or treated) on a literature review and calibrated the estimates against the most recent data on HBV‐related deaths (2019; estimated 42713) and the incidence of HBV‐attributable hepatocellular carcinoma (2017; estimated 2174 incident cases and projected incidence (2018–2021) of 2451–2832 cases14; estimated 22% of hepatocellular carcinoma cases attributable to chronic HBV infection15) (Supporting Information, table 4).
The population prevalence of chronic hepatitis B among people born before 2000 without diagnosed hepatitis B was estimated to be 0.45%, based on the modelled prevalence of HBV infections in Australia (0.87%), the proportion of people with chronic hepatitis B who have been diagnosed with the condition (73%), and the estimated proportion of the population born before 2000 (76.0% in 2020) (Supporting Information, supplementary methods).
Our analysis assumed the health care funders’ perspective, which is most relevant to policy makers considering universal screening; further, data for analysis from a societal perspective are not available for Australia. Total costs included relevant direct medical costs in each state and the costs of HBV testing (Supporting Information, table 5); our main data sources were the Medicare Benefits Schedule (http://www.mbsonline.gov.au) and the Pharmaceutical Benefit Scheme (https://www.pbs.gov.au). Health utility was assessed as quality‐adjusted life‐years (QALYs); utility values for each disease state were obtained from published empirical studies (Supporting Information, table 6). Costs and health utility were each discounted by 5% per year, consistent with national guidelines for discounting in health economic evaluations.16
Scenarios
Three scenarios were compared:
- Scenario 1. Status quo; no change to current HBV testing practice.
- Scenario 2. Universal screening strategy, with the aim of achieving the WHO diagnosis target by 2030 (90% of people with chronic hepatitis B are diagnosed; 2020 level: 73%3), assuming opportunistic (general practitioner‐initiated) screening for HBsAg (the primary marker for current HBV infection). Only the test costs are included in the analysis; ie, neither opportunity cost in terms of health care provider time nor additional Medicare service cost. Community and health service engagement costs and costs associated with education for implementing and monitoring the screening program were not included.
- Scenario 3. Universal screening strategy, with the aim of achieving the WHO diagnosis target by 2030, and also ensuring that 50% of people with chronic hepatitis B are receiving appropriate clinical management (but not necessarily pharmacological treatment) by 2030 (2020 level: 22.6%3).
Outcomes
The main outcomes were the projected care cascade for people with chronic hepatitis B, the incidence of HBV‐related hepatocellular carcinoma during 2020–2030, the cumulative number of HBV‐related deaths during 2020–2030, costs, and health utility, and the incremental cost‐effectiveness ratios (ICERs) for scenarios 2 and 3 (compared with scenario 1). The notional willingness‐to‐pay threshold, indicating intervention cost‐effectiveness, was set at $50 000 per QALY gained.
Sensitivity analyses
In one‐way sensitivity analyses, we tested the effect on the ICERs of scenarios 2 and 3 of varying specific parameters, and calculated the threshold values for test positivity rate and additional costs (scenario 3 only) that would allow ICERs of less than $50 000 per QALY gained. We also conducted a probabilistic multivariate uncertainty analysis (1000 iterations) with values of all parameters randomly selected from their ranges (normal distribution assumed for all parameters unless noted otherwise); the confidence intervals for the main analysis outcomes were used as the interquartile range for the corresponding model runs.
Ethics approval
We did not seek ethics approval for our analysis of publicly available data.
Results
In scenario 1, there were 413 HBV‐related deaths in 2021 (interquartile range [IQR], 318–906 deaths) and 709 deaths in 2030 (IQR, 548–912 deaths); in 2030, 82% of people with chronic hepatitis B (IQR, 80–86%) had been diagnosed, 35% (IQR, 33–38%) received appropriate clinical management, and 20% (IQR, 20–25%) were receiving treatment. Eighty deaths (IQR, 41–127 deaths) were averted during 2020–2030 in scenario 2, and 315 HBV‐related deaths (IQR, 211–454 deaths) in scenario 3. Scenario 2 cost $84 million (IQR, $41–106 million) more than scenario 1 during 2020–2030 (+8%), yielding an ICER of $104 921 (IQR, $49 587–107 952) per QALY gained. Scenario 3 cost $263 million (IQR, $214–316 million) more than scenario 1 during 2020–2030 (+24%), yielding an ICER of $47 341 (IQR, $32 643–58 200) per QALY gained (Box 1).
Sensitivity analyses
The cost‐effectiveness of scenarios 2 and 3 increased with test positivity rate; rates of 2.26% (scenario 2) or 0.35% (scenario 3) were required to achieve an ICER of $50 000 per QALY gained (Box 2, Box 3).
For the main analysis, we assumed that HBsAg tests were used for universal screening ($15.02 per test, MBS‐subsidised, weighted by level of benefit; Supporting Information, p. 2). If a three‐marker HBV test (HBsAg, anti‐HBsAg, anti‐HBcAg) was used ($38.93 per test after subsidy; Supporting Information, p. 3), neither scenario 2 nor 3 would be cost‐effective. If a point‐of‐care HBsAg test (available; not currently subsidised by Medicare) was used ($11.67 per test),17 scenario 3 but not scenario 2 would be cost‐effective (Box 2, Box 3).
For the main analysis, we assumed that universal screening involved no costs other than test unit costs. Scenario 3 would remain cost‐effective should additional costs (eg, for health care provider time) of no more than $4.02 per test be incurred.
In the sensitivity analysis, the ICERs of scenarios 2 and 3 were most sensitive to change in health utility for people receiving treatment (compared with people in the same disease state not receiving treatment). This treatment effect is distinct from the impact of treatment on disease progression. Compared with a base estimate of a 10% increase in health utility, the ICER for scenario 2 was $60 439 per QALY gained with a 20% increase in utility and $397 371 per QALY gained with 0% increase in utility; for scenario 3, the ICERs was $26 777 per QALY gained with a 20% increase in utility and $204 039 per QALY gained with 0% increase in utility (Supporting Information, table 8).
Discussion
Our findings suggest that universal HBV screening with the aim of diagnosing chronic hepatitis B in 90% of people with chronic hepatitis B in Australia by 2030, would incur an additional $84 million (IQR, $41–106 million) in costs during 2020–2030 (compared with current practice), avert 80 HBV‐related deaths (IQR, 41–127 deaths), and lead to 804 QALYs gained (IQR, 459–1260 QALYs gained), with an ICER of $104 921 (IQR, $49 587–107 952) per QALY gained. If, in addition, 50% of people with chronic hepatitis B were to receive appropriate clinical management, the extra cost would be $263 million (IQR, $214–316 million), 315 HBV‐related deaths would be prevented (IQR, 211–454 deaths), and 5550 QALYs would be gained (IQR, 4418–7741 QALYs gained), yielding an ICER of $47 341 (IQR, $32 643–58 200) per QALY gained.
Our findings indicate that ensuring people with chronic hepatitis B receive appropriate clinical management is critical for the cost‐effectiveness of a universal screening strategy. Almost four times as many HBV‐related deaths were averted in scenario 3 as in scenario 2; that is, higher testing and diagnosis rates without increasing the rate of clinical management was not cost‐effective.
However, the cost‐effectiveness of scenario 3, with its combination of both increased screening and clinical management, was sensitive to the inclusion of extra costs; should they exceed $4.02 per person tested, the intervention was no longer cost‐effective. Extra costs would arise were testing not entirely undertaken on an opportunistic basis (eg, required longer general practitioner consultations), were financial support needed for general practitioners to provide testing, or if opportunistic testing did not achieve high coverage of people with hepatitis B who do not visit general practitioners. Funding of outreach services and community awareness programs could also cause additional costs.
The test unit cost and type of test influenced the cost‐effectiveness of universal screening. The three‐marker test is recommended for HBV screening, as it not only identifies current and past infections (apart from occult infections), but also people who would benefit from vaccination, unlike HBsAg screening alone.9 However, neither scenario 2 nor 3 would be cost‐effective were the three‐marker HBV test panel used for universal screening.
The test positivity rate also influenced cost‐effectiveness. The base estimated rate (0.45%) is conservative and subject to uncertainty about HBV infection prevalence, the proportion of infected people who have been diagnosed, and the proportion of the target population who have already been tested. A recent Global Burden of Disease HBV modelling study reported a higher estimated prevalence of HBV infections in Australia (2.1% in 2019).18 Further, we may have overestimated the proportion of infected people diagnosed (73% in 2020), as duplicate notifications of hepatitis B to the National Notifiable Diseases Surveillance System are possible. Further, the proportion of the target population previously tested is unknown. In light of these factors, the test positivity rate could exceed 2% (for example: 2.17% if hepatitis B prevalence is 2%, 50% of people aged 20 years or more have already been tested, and only 60% of people with chronic hepatitis B have been diagnosed), making universal screening more cost‐effective than we estimated.
The cost‐effectiveness of universal screening would also vary between states because of differences in hepatitis B prevalence (estimated 0.28% to 1.84% by primary health network area) and proportion of people with an HBV infection diagnosis (estimated 57% to 79% by state).3 It could be more cost‐effective to target specific groups with higher HBV infection prevalence for screening, but this could increase the implementation costs.
Limitations
First, the quality of our projections depends on that of the model inputs. We used a modelled estimate of population prevalence because actual HBV prevalence data were unavailable, but the estimate was based on national population structure and notification data; further, we tested a wide range of parameter values in our sensitivity analysis and uncertainty analysis. Second, liver transplantation was not a compartment in our model, but rates of HBV‐related liver transplantation are low in Australia19 and would have only a small impact on the ICERs of the modelled scenarios. Third, the analysis assumed that the test positivity rate was constant during the study period, which may not be the case in practice. We did not examine the feasibility of universal screening; it may not achieve the 90% diagnosis target if universal screening is incomplete. Fourth, late effects of coronavirus disease 2019 (COVID‐19)‐related travel restrictions might influence hepatitis B epidemiology in Australia, as most new cases of chronic hepatitis B are diagnosed in immigrants.3 We did not directly assess the impact of COVID‐19, but tested several values for the annual increase in the number of people with chronic hepatitis B (range, 0–6000) in our sensitivity analysis (Supporting Information, table 9). Finally, our ICERs should be interpreted cautiously because additional costs (eg, health care provider time, implementation costs, additional costs for screening specific groups) were not included in the analysis. Further, the ICERs are highly sensitive to the assumption of the greater utility of treatment compared with no treatment.
Conclusion
We have previously reported the cost‐effectiveness of achieving national and WHO hepatitis B elimination targets in Australia,5 but specific mechanisms for achieving these targets have not been investigated. One approach, universal HBV screening to increase diagnosis rates, is being discussed both in Australia and overseas.20,21,22 Our epidemiological model, calibrated using the most recent Australian data and taking COVID‐19 pandemic‐related migration changes into account, provides evidence that universal screening could be cost‐effective for reaching the WHO diagnosis target in Australia by 2030, but only if combined with high rates of clinical management and low cost testing. Targeted testing of people in groups in which HBV prevalence is higher could increase cost‐effectiveness.
Box 1 – Care cascade, health impacts, costs, and cost‐effectiveness of three hepatitis B virus (HBV) testing scenarios*
|
Outcome |
Scenario 1 |
Scenario 2 |
Scenario 3 |
||||||||||||
|
|
|||||||||||||||
|
Care cascade for people with chronic hepatitis B (2030): proportion (IQR) |
|
|
|
||||||||||||
|
Diagnosed |
82% (80–86%) |
90% (88–93%) |
90% (88–93%) |
||||||||||||
|
Receiving appropriate clinical management |
35% (33–38%) |
37% (35–39%) |
50% (47–52%) |
||||||||||||
|
Receiving pharmacological treatment |
20% (20–25%) |
21% (21–26%) |
27% (27–33%) |
||||||||||||
|
Health impact (IQR) |
|
|
|
||||||||||||
|
New hepatocellular carcinoma cases (2030) |
633 (457–802) |
625 (452–788) |
593 (426–741) |
||||||||||||
|
Deaths attributed to chronic hepatitis B (2030) |
709 (548–912) |
697 (535–896) |
649 (506–830) |
||||||||||||
|
Cumulative HBV‐related deaths (2020–2030) |
6093 (5634–8235) |
6013 (5553–8101) |
5788 (5372–7818) |
||||||||||||
|
HBV‐related deaths averted (v scenario 1) |
— |
80 (41–127) |
315 (211–454) |
||||||||||||
|
Reduction in HBV‐attributable mortality (v scenario 1) |
— |
1% (1–2%) |
5% (4–6%) |
||||||||||||
|
Costs: millions (IQR) |
|
|
|
||||||||||||
|
Discounted total costs (2020–2030) |
$1105 ($992–1310) |
$1189 ($1065–1385) |
$1368 ($1233–1588) |
||||||||||||
|
Difference in cost (v scenario 1) (IQR) |
— |
$84 ($41–106) |
$263 ($214–316) |
||||||||||||
|
Health utility: quality‐adjusted life years [QALYs] (IQR) |
|
|
|
||||||||||||
|
QALYs (2020–2030) |
1 693 678 (1 633 716–1 822 180) |
1 694 483 (1 634 434–1 823 397) |
1 699 229 (1 640 752–1 829 583) |
||||||||||||
|
QALYs gained (2020–2030) (v scenario 1) |
— |
804 (459–1260) |
5550 (4418–7741) |
||||||||||||
|
Incremental cost effectiveness ratio (cost per QALY gained) |
— |
$104 921 (49 587–107 952) |
$47 341 (32 643–58 200) |
||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. * Scenario 1: status quo; scenario 2: universal screening; scenario 3: universal screening and clinical management of 50% of people with chronic hepatitis B. |
|||||||||||||||
Box 2 – The incremental cost effectiveness ratio (ICER) for two universal hepatitis B virus (HBV) screening scenarios, by test positivity rate and unit screening cost*
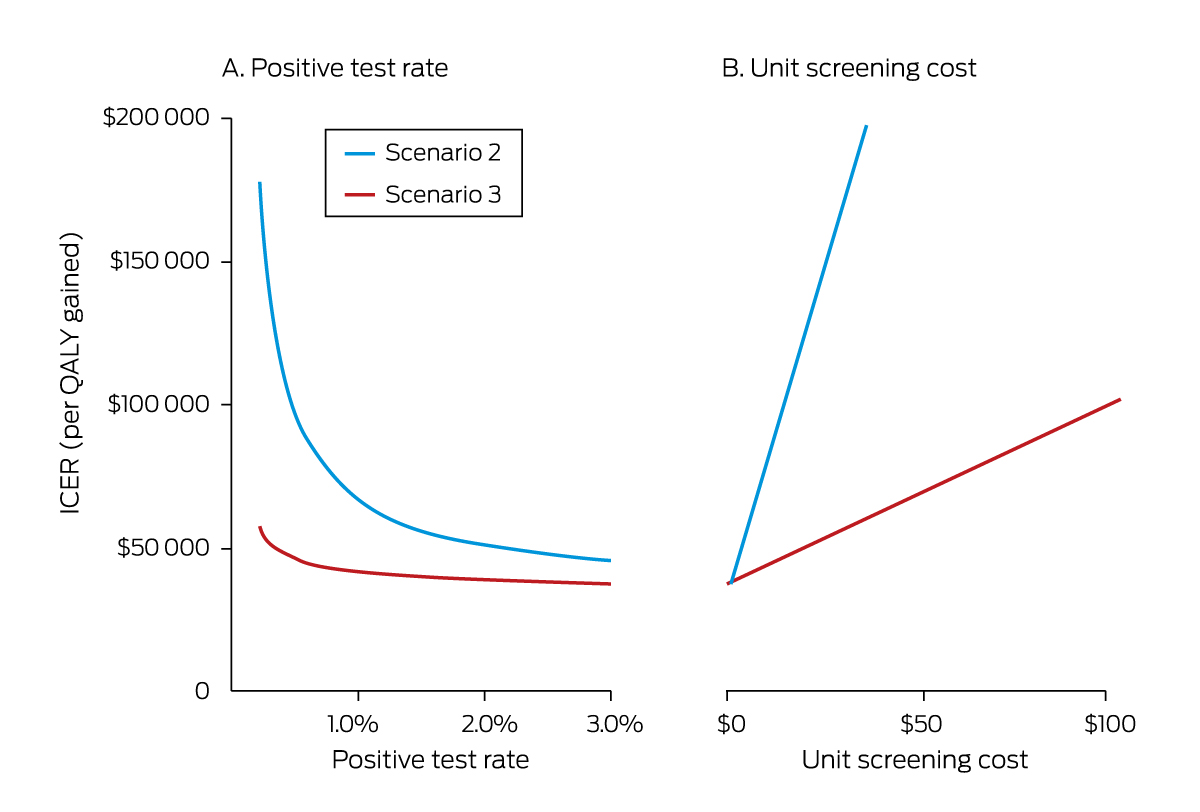
* Scenario 2: universal screening; scenario 3: universal screening and clinical management of 50% of people with chronic hepatitis B. In panel A, the unit screening cost (testing cost only) is $15.02 per person tested; in panel B, the test positivity rate of 0.45%. The effects on the ICER of scenario 3 of varying both the test positivity rate and the unit screening cost are reported in the Supporting Information, table 7.
Box 3 – The influence of screening test cost, test positivity rate, and additional costs on the incremental cost effectiveness ratio (ICER) for two universal hepatitis B virus (HBV) screening scenarios*
|
|
|
ICER, per QALY gained (95% CI) |
|
||||||||||||
|
Testing parameter/assumption |
Value |
Scenario 2 |
Scenario 3 |
Notes |
|||||||||||
|
|
|||||||||||||||
|
Assumptions for main analysis |
|
$104 921 |
$47 341 |
|
|||||||||||
|
Screening test: standard HBsAg test |
$15.02 |
|
|
HBsAg testing, Medicare‐subsidised (MBS item number 69475 weighted by level of benefit†) |
|||||||||||
|
Testing positivity rate |
0.45% |
|
|
Estimated hepatitis B prevalence among people without a hepatitis B diagnosis born before 2000 |
|||||||||||
|
Additional cost per test |
$0 |
|
|
Opportunistic testing |
|||||||||||
|
Testing assumptions ‡ |
|
|
|
|
|||||||||||
|
Screening test: three HBV marker tests |
$38.93 |
$213 749 (79 134– 214 616) |
$63 110 |
Unit costs for three HBV markers ($40.55; MBS‐subsidised; MBS item number 69481) |
|||||||||||
|
Screening test: HBsAg rapid test |
$11.67 |
$89 652 |
$45 129 |
Online purchase price (16 Feb 2022). |
|||||||||||
|
Test positivity rate |
0.87% |
$71 645 |
$42 520 |
Assumed hepatitis B prevalence in general population |
|||||||||||
|
Test positivity rate |
2.17% |
$50 415 |
$42 603 |
Assumed hepatitis B prevalence of 2%, 50% of people aged 20 years and over ever tested for HBsAg, and only 60% of people with chronic hepatitis B had been diagnosed with the condition |
|||||||||||
|
Test positivity rate |
2.26% |
$50 000 |
— |
Threshold calculation (scenario 2) |
|||||||||||
|
Test positivity rate |
0.35% |
— |
$50 000 |
Threshold calculation (scenario 3) |
|||||||||||
|
Additional costs per test |
$4.02 |
— |
$50 000 |
Threshold calculation (scenario 3) |
|||||||||||
|
|
|||||||||||||||
|
CI = confidence interval; HBV = hepatitis B virus; ICER = incremental cost‐effectiveness ratio; MBS = Medical Benefits Schedule; QALY = quality‐adjusted life year. * Scenario 2: universal screening; scenario 3: universal screening and clinical management of 50% of people with chronic hepatitis B. † Details in Supporting Information, supplementary methods. ‡ Each applied separately. |
|||||||||||||||
Received 28 April 2022, accepted 21 November 2022
- Yinzong Xiao1
- Margaret E Hellard1,2
- Alexander J Thompson3,4
- Christopher Seaman1,5
- Jess Howell1,3
- Nick Scott1,5
- 1 The Burnet Institute, Melbourne, VIC
- 2 The Alfred Hospital, Melbourne, VIC
- 3 St Vincent's Hospital, Melbourne, VIC
- 4 The University of Melbourne, Melbourne, VIC
- 5 Monash University, Melbourne, VIC
We acknowledge support from the Victorian Operational Infrastructure Support Program received by the Burnet Institute. Margaret Hellard is supported by a National Health and Medical Research Council (NHMRC) Investigator Grant (GNT1194322) and an NHMRC program grant (GNT1132902). Alexander J Thompson has received an NHMRC program grant (GNT1132902) and MRFF Practitioner Fellowship (1142976). Jessica Howell is supported by a University of Melbourne CR Roper Faculty Fellowship and an NHMRC Program Grant. Nick Scott holds an NHMRC fellowship (GNT2009408). Christopher Seaman is supported by an Australian Government Research Training Program scholarship.
Margaret Hellard receives funding from Gilead Sciences and Abbvie for investigator‐initiated research. Margaret Hellard, Alexander J Thompson, and Jess Howell have received unrelated investigator‐initiated research grants from Gilead Sciences, AbbVie, Merck/MSD, and Bristol Myers Squibb. Alexander J Thompson has received consulting fees from Gilead, Abbvie, Roche, BMS, Merck, Immunocore, Janssen, Assembly Biosciences, Arbutus, Eisai, Ipsen and Bayer, speaker fees from Gilead Sciences, and investigator‐initiated grants from Gilead Sciences. Jess Howell has received speaker fees and investigator‐initiated grants from Gilead Sciences. Nick Scott has received unrelated research grants from Gilead Sciences.
- 1. World Health Organization. Global hepatitis report, 2017. 19 Apr 2017. https://www.who.int/publications/i/item/9789241565455 (viewed Jan 2020).
- 2. World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. 15 July 2021. https://www.who.int/publications/i/item/9789240027077 (viewed July 2021).
- 3. MacLachlan J, Stewart S, Cowie B. Viral hepatitis mapping project: national report 2020. Sydney: Australasian Society for HIV, Viral Hepatitis, and Sexual Health Medicine, 2021. https://ashm.org.au/wp‐content/uploads/2022/04/ASHM_ViralHepReport_2020_WEB_final.pdf (viewed Feb 2022).
- 4. McCulloch K, Romero N, MacLachlan J, et al. Modelling progress toward elimination of hepatitis B in Australia. Hepatology 2019; 71: 1170‐1181.
- 5. Xiao Y, Howell J, van Gemert C, et al. Enhancing the hepatitis B care cascade in Australia: a cost‐effectiveness model. J Viral Hepat 2020; 27: 526‐536.
- 6. World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. June 2016. https://apps.who.int/iris/handle/10665/246177 (viewed Jan 2020).
- 7. Australasian Society for HIV Medicine, Viral Hepatitis and Sexual Health Medicine. National hepatitis B testing policy 2020 (version 1.2). https://testingportal.ashm.org.au/national‐hbv‐testing‐policy (viewed Feb 2022).
- 8. Allard NL, MacLachlan JH, Tran L, et al. Time for universal hepatitis B screening for Australian adults. Med J Aust 2021; 215: 103‐105. https://www.mja.com.au/journal/2021/215/3/time‐universal‐hepatitis‐b‐screening‐australian‐adults
- 9. Lubel JS, Strasser SI, Thompson AJ, et al. Australian consensus recommendations for the management of hepatitis B. Med J Aust 2022; 216: 478‐486. https://www.mja.com.au/journal/2022/216/9/australian‐consensus‐recommendations‐management‐hepatitis‐b
- 10. Wallace J, Hajarizadeh B, Richmond J, McNally S. Challenges in managing patients in Australia with chronic hepatitis B: the General Practitioners’ perspective. Aust N Z J Public Health 2013; 37: 405‐410.
- 11. van Gemert C, Howell J, Wang J, et al. Knowledge and practices of chronic hepatitis B virus testing by general practitioners in Victoria, Australia, 2014–15. Aust Fam Physician 2017; 46: 683‐689.
- 12. Toy M, Hutton D, Harris AM, et al. Cost‐effectiveness of 1‐time universal screening for chronic hepatitis B infection in adults in the United States. Clin Infect Dis 2022; 74: 210‐217.
- 13. Romero N, McCulloch K, Allard N, et al. National surveillance for hepatitis B indicators: measuring the progress towards the targets of the national hepatitis B strategy. Annual report 2019. Melbourne: WHO Collaborating Centre for Viral Hepatitis; the Doherty Institute, 2020. https://www.doherty.edu.au/uploads/content_doc/National_Surveillance_for_Hepatitis_B_Indicators_2019_final.pdf (viewed Feb 2022).
- 14. Australian Institute of Health and Welfare. Cancer data in Australia (Cat. no. CAN 122). Updated 8 June 2021 (note: this version no longer accessible). https://www.aihw.gov.au/reports/cancer/cancer‐data‐in‐australia/data (viewed June 2021).
- 15. Hong TP, Gow PJ, Fink M, et al. Surveillance improves survival of patients with hepatocellular carcinoma: a prospective population‐based study. Med J Aust 2018; 209: 348‐354. https://www.mja.com.au/journal/2018/209/8/surveillance‐improves‐survival‐patients‐hepatocellular‐carcinoma‐prospective
- 16. Pharmaceutical Benefits Advisory Committee. Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee; version 5.0. Sept 2016. https://pbac.pbs.gov.au/content/information/files/pbac‐guidelines‐version‐5.pdf (viewed Mar 2022).
- 17. STI Test Kits. iCare hepatitis B rapid test kit. https://www.buystdtestkits.com/products/hepatitis‐b‐rapid‐test‐kit (viewed Feb 2022).
- 18. GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol 2022; 7: 796‐829.
- 19. Australia and New Zealand Liver and Intestinal Transplant Registry 32nd annual report. Report on liver and intestinal transplantation activity to 31/12/2020. Dec 2021. https://www.anzlitr.org/wp‐content/uploads/2021/12/32ndANZLITR_AnnualReport.pdf (viewed Feb 2022).
- 20. Allard NL, MacLachlan JH, Tran L, et al. Time for universal hepatitis B screening for Australian adults. Med J Aust 2021; 215: 103‐105. https://www.mja.com.au/journal/2021/215/3/time‐universal‐hepatitis‐b‐screening‐australian‐adults
- 21. Toy M, Hutton D, Harris AM, et al. Cost‐effectiveness of 1‐time universal screening for chronic hepatitis B infection in adults in the United States. Clin Infect Dis 2022; 74: 210‐217.
- 22. Su S, Wong WC, Zou Z, et al. Cost‐effectiveness of universal screening for chronic hepatitis B virus infection in China: an economic evaluation. Lancet Glob Health 2022; 10: e278‐e287.





Abstract
Objectives: To assess the impact on diagnosis targets, cost, and cost‐effectiveness of universal hepatitis B screening in Australia.
Design: Markov model simulation of disease and care cascade progression for people with chronic hepatitis B in Australia.
Setting: Three scenarios were compared: 1. no change to current hepatitis B virus (HBV) testing practice; 2. universal screening strategy, with the aim of achieving the WHO diagnosis target by 2030 (90% of people with chronic hepatitis B diagnosed), based on opportunistic (general practitioner‐initiated) screening for HBsAg; 3. universal screening strategy, and also ensuring that 50% of people with chronic hepatitis B are receiving appropriate clinical management by 2030.
Main outcome measures: Projected care cascade for people with chronic hepatitis B, cumulative number of HBV‐related deaths, intervention costs, and health utility (quality‐adjusted life‐years [QALYs] gained during 2020–2030). An incremental cost‐effectiveness ratio (ICER) threshold (v scenario 1) of $50 000 per QALY gained was applied.
Results: Compared with scenario 1, 80 HBV‐related deaths (interquartile range [IQR], 41–127 deaths) were averted during 2020–2030 in scenario 2, 315 HBV‐related deaths (IQR, 211–454 deaths) in scenario 3. Scenario 2 cost $84 million (IQR, $41–106 million) more than scenario 1 during 2020–2030 (+8%), yielding an ICER of $104 921 (IQR, $49 587–107 952) per QALY gained. Scenario 3 cost $263 million (IQR, $214–316 million) more than scenario 1 during 2020–2030 (+24%), yielding an ICER of $47 341 (IQR, $32 643–58 200) per QALY gained. Scenario 3 remained cost‐effective if the test positivity rate was higher than 0.35% or the additional costs per person tested did not exceed $4.02.
Conclusions: Universal screening for hepatitis B will be cost‐effective only if the cost of testing is kept low and people receive appropriate clinical management.