Coronavirus disease 2019 (COVID‐19) emerged in Wuhan, China in late 2019 and the World Health Organization declared a global health emergency on 31 January 2020. Currently, 10–15% of those affected develop severe disease, and worldwide mortality is around 6%.1 Transmission of the causal virus — severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) — is primarily by contact with droplets, fomites and to a lesser degree, aerosol generation.2 For health care workers, aerosol generation during airway management is a significant concern. In cardiothoracic surgery there are particular procedures that are high risk for aerosol generation and hence transmission of COVID‐19. In order to protect staff, it is important to minimise these procedures if possible, or ensure that they are conducted in a suitable environment with appropriate personal protective equipment (PPE). While screening tools are available to assess the risk of a patient contracting COVID‐19, transmission can occur from asymptomatic patients, albeit at low risk. The Royal Australasian College of Surgeons has published guidelines for the management of surgical patients during the COVID‐19 pandemic.3 Here, we discuss issues specific to cardiothoracic surgery; key recommendations are summarised in Box 1.
Methods
A survey conducted by the Anaesthetic Continuing Education Cardiac Thoracic Vascular and Perfusion Special Interest Group committee on 12 April 2020 revealed wide variability in the management of cardiothoracic surgical patients during the COVID‐19 pandemic. Following discussions with the Australian and New Zealand Society of Cardiac and Thoracic Surgeons committee, it was felt that despite recommendations in airway management and ventilation, there was limited information and guidance for the management of aerosol generation during cardiothoracic surgery and the potential for prolonged and ongoing exposure during these procedures. A need for guidance in this subspecialty group during the pandemic was identified.
A panel of six experts (three cardiothoracic anaesthetists and three cardiothoracic surgeons) was assembled from different states and hospitals across Australia to review national guidance, consensus recommendations and current literature on aerosol generation relevant to cardiothoracic surgery and the management of patients during the COVID‐19, severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks.
Articles reviewed were of low or very low quality evidence according to Grading of Recommendations, Assessment, Development and Evaluation (GRADE) criteria, consisting mainly of observational data and expert opinion. Our recommendations were based on the principles outlined in these publications combined with expert opinion and experience in the subspecialty of cardiothoracic surgery.
Our recommendations were then referred to the Anaesthetic Continuing Education Cardiac Thoracic Vascular and Perfusion Special Interest Group and Australian and New Zealand Society of Cardiac and Thoracic Surgeons executive committees, comprising cardiothoracic anaesthetists and surgeons from across Australia and New Zealand, for review and comment. The final consensus statement has been endorsed by these bodies. We acknowledge that as evidence and knowledge regarding COVID‐19 continues to evolve, guidance may change.
Recommendations
Personal protective equipment
Guidelines regarding PPE have been produced by individual state government health authorities (eg, the New South Wales Clinical Excellence Commission and the Victorian Department of Health and Human Services) in response to the COVID‐19 outbreak.4,5
PPE type is classified as standard, contact, droplet or airborne precautions. Formal training is essential and donning and doffing procedures should be strictly followed. N95 and P2 masks should be fit tested and fit checked each time they are applied.6 The most effective protection is frequent and effective hand hygiene.7
Risk stratification
Risk stratification is paramount in patients presenting for cardiothoracic surgery. The definitions for confirmed, probable and suspected cases can be found in the Communicable Diseases Network Australia national guidelines.8
High risk patients include:
- patients with confirmed COVID‐19 infection;
- symptomatic patients without a negative swab or clearance from an infectious disease specialist or department;
- asymptomatic patients with epidemiological risk factors; and
- unconscious patients unable to provide risk screening information, specifically patients presenting with trauma and out‐of-hospital cardiac arrest.
The merit of performing routine reverse transcriptase polymerase chain reaction testing of all patients planned for cardiothoracic surgery is debatable. Interpreting the result depends on the accuracy of the test and the pre‐test probability of disease. It may allow positive patients to be identified and timing of surgery to be reconsidered due to the high specificity.9 A single negative test result, however, should not be used as to rule out patients with strongly suggestive symptoms. A systematic review of the accuracy of COVID‐19 tests reported false negative rates of between 2% and 54%.10
Currently, risk stratification according to the patient's clinical circumstances and local COVID‐19 epidemiology is the most appropriate strategy.
Scheduling of cardiothoracic surgery
The majority of cardiothoracic surgical cases are performed for prognostic reasons. The timing of these procedures will depend on a balance between the benefit to the patient, the risk of SARS‐CoV‐2 infection, resource availability, and local government restrictions.
As COVID‐19 can present with both cardiac and respiratory symptoms, it is vital that patients are appropriately screened and that a differential diagnosis of COVID‐19 be considered. The Royal Australasian College of Surgeons recommends that positive or suspected patients be managed conservatively, if possible, with their surgery delayed.3 If emergency surgery is required then patients should be risk assessed. It should be remembered that acute coronary syndrome is a common cardiac manifestation of COVID‐19.11 A statement from the Australian and New Zealand Society of Cardiac and Thoracic Surgeons and the Cardiac Society of Australia and New Zealand provides guidance on the management of such patients.11
Data on the risk of COVID‐19‐positive patients undergoing cardiothoracic surgery are limited, although high peri‐operative mortality is suggested. A study from Wuhan in patients undergoing non‐cardiac elective surgery during the incubation period of COVID‐19 demonstrated a 44% intensive care requirement and 20% mortality.12
Mechanics of aerosols and the spread of SARS‐CoV‐2
Most SARS‐CoV‐2 spread is likely to be direct or indirect (fomite) contact or droplet spread. The World Health Organization indicates that airborne spread may occur but only during aerosol‐generating procedures.2
Clinical risk data on aerosol risk of transmission are summarised in a systematic review of severe acute respiratory syndrome,13 although the ten studies included were classified by GRADE as very low quality evidence. Procedures presenting an increased risk of transmission included tracheal intubation, non‐invasive ventilation, manual ventilation before intubation and tracheostomy.
The mechanisms responsible for the generation of aerosolised particles require disruption of the surface tension of the respiratory tract lining fluid.14 This may be caused by high shear forces or manual or mechanical disruption during lung surgery.
Aerosol generation and cardiothoracic surgery
A number of procedures are at a high risk of aerosol generation during cardiothoracic surgery. These include bag–mask ventilation; tracheal intubation; tracheal extubation; bronchoscopy; lung isolation; continuous positive airway pressure; lung recruitment or reinflation; lung injury, air leak, bronchopleural fistula or decortication; and chest drain management.
The magnitude and spread of SARS‐CoV‐2 will vary greatly over time and geographical location and, therefore, advice may change with respect to low risk patients undergoing procedures at high risk of aerosol generation, depending on the community transmission and the potential for asymptomatic patients attending for procedures.
Due to the number and frequency of aerosolising procedures performed during thoracic surgery, we believe this surgery, in particular, has the potential to generate high volumes of prolonged and ongoing aerosol generation. As such, in areas or times when risk of community transmission is high, it may be reasonable to adopt airborne precautions for all thoracic patients irrespective of COVID‐19 status to protect staff.15
Management of such patients should be in keeping with national, regional and institutional policies and with recognition of the requirement to conserve PPE.
Operating theatre set‐up
There should be a designated operating room to manage COVID‐19 patients, with a protocol for patient flow to and from the operating room.16 Negative (or neutral) pressure rooms are preferred. Dirty and clean buffer zones should be observed and staff and equipment should be kept to a minimum. Disposable equipment should be used where available and clean runners should be available to provide additional instruments, drugs and equipment.
Team briefings are important. These should aim to introduce the team, discuss the surgery, identify aerosolising procedures, and review protocols. Communication may be difficult in PPE and this should be recognised.
Anaesthesia
The peak risk period during cardiac surgical procedures is intubation and extubation. The Safe Airway Society has produced guidelines for airway management in the COVID‐19 environment.17 Intubation and extubation processes should follow these accepted guidelines to minimise aerosolisation. There should be a wait time, if possible, for the aerosol risk to subside, with a recommendation of three to five air changes (about 15 minutes).15 When transferring intubated patients to the intensive care unit, the number and duration of breathing circuit disconnections should be minimised, with processes to prevent aerosols as per Safe Airway Society guidance.17
Lung isolation and single lung ventilation
Lung isolation can be achieved by a number of measures. Options include left‐ or right‐sided double lumen tubes, bronchial blockers, or a single lumen tube advanced into the non‐operative lung.18
The European Association of Cardiothoracic Anaesthesiology has published recommendations for airway management in thoracic anaesthesia.19 A survey conducted by the Association showed that the choice of lung isolation management is likely to be influenced by the intubation status of the patient, predicted airway difficulty and individual preference.19
Many steps involved in the achievement and troubleshooting of lung isolation are high aerosolising procedures. There are a number of adaptations that we suggest be adopted to minimise this.
Intubation should be undertaken as per Safe Airway Society guidance, with an experienced thoracic anaesthetist. Clinical assessment of double lumen tube placement can be performed by inspecting and auscultating, while sequentially clamping the tracheal and bronchial arms without disconnection. This may avoid the need for bronchoscopy.
Another option is to confirm lung ventilation with ultrasound, looking for lung sliding (ventilation) and absence of lung sliding and lung pulse (no ventilation but no pneumothorax).20
If bronchoscopy is required for positioning or troubleshooting, this should be performed with the ventilator disabled, without positive pressure on the circuit, and with the patient adequately paralysed.
Double lumen tubes with embedded cameras may reduce the requirement for bronchoscopy.21 There will be a learning curve associated with these devices. Although they may reduce the aerosol risk during placement, they are not a substitute for the wider use of bronchoscopy during thoracic surgery.
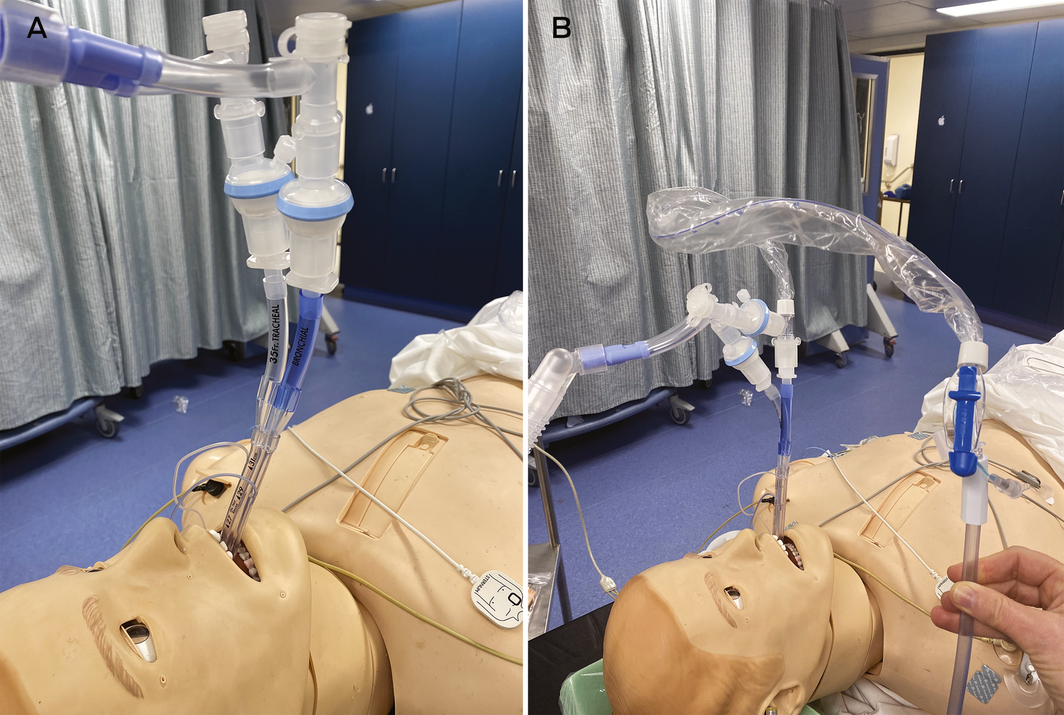
During lung isolation, we recommend that viral filters be placed on each limb of the double lumen tube to protect against aerosols during deflation and any leak of ventilated gas. Paediatric filters may be useful to reduce the weight and kinking of the tube (Box 2, A). Application of continuous positive airway pressure to the non‐ventilated lung, if required, should be performed with the filter in place.
Suctioning of the lung is often required and we recommend using closed in‐line suction. This should be inserted with the ventilator disabled and the tube clamped to minimise aerosol generation (Box 2, B). The patient should be adequately paralysed to prevent coughing. Self‐sealing suction‐safe connectors can also be placed to minimise circuit disconnections.
Air leak during thoracic surgery
Any thoracic surgical procedure where there is presence or creation of alveolar, parenchymal or bronchopleural leak should be considered an open airway and high risk for aerosol generation. This includes lung resection surgery, decortication and pneumothorax surgery. While many surgeons use lung resection techniques that minimise an open bronchial tree or alveolar leak, these cannot guarantee freedom from aerosols. Pulmonary decortication is frequently associated with alveolar air leak and should be considered very high risk. Airborne PPE should be employed for these procedures.
Procedural bronchoscopy
Bronchoscopy is at high risk for aerosol generation and should be minimised.17 We recommend all bronchoscopy should be videoscopic, with airborne PPE employed. Rigid bronchoscopy is very high risk and should be avoided.
Chest drain management
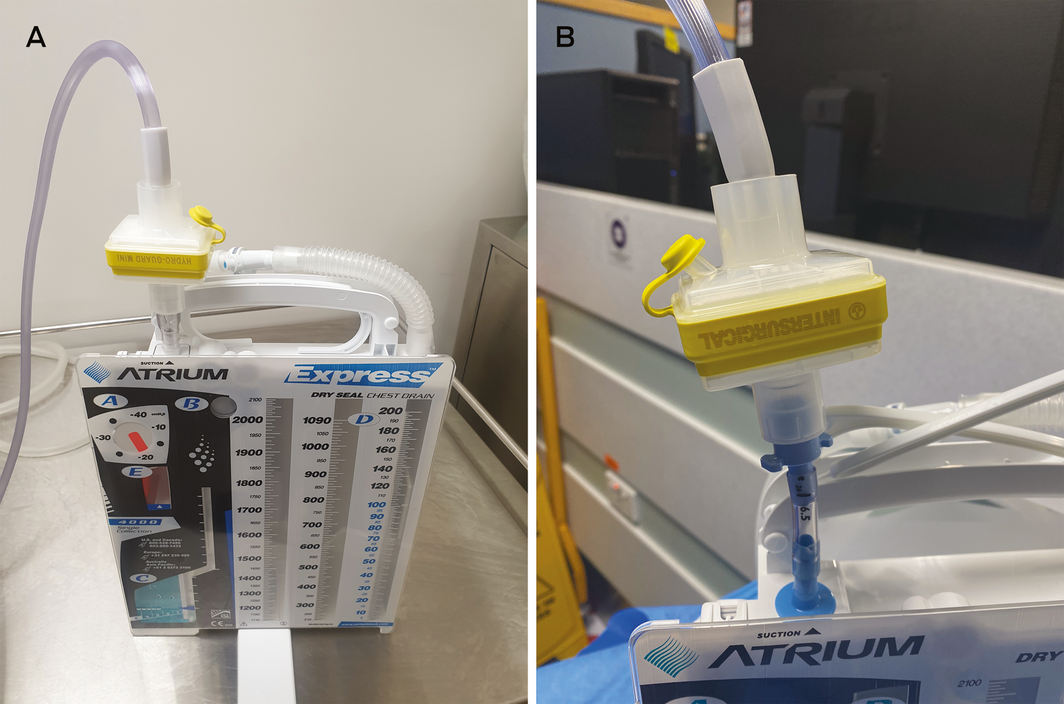
If virus is present in the pleural space then there is risk of aerosolisation at the chest drain exhaust.22 Chest drain insertion, even by careful closed technique, is a risk. When an underwater drain bottle is on suction, the risk is minimal; however, if the bottle is open to air then risk exists, which will be higher with any air leak.
To minimise risk, a viral filter can be applied to the exhaust vent.22 Suction can then be connected to the filter exhaust (Box 3). The resistance of the viral filter up to a flow of 30 L/min has been found to be trivial, correlating with clinical experience that the filter does not impede airflow out of the chest.23 It is important, however, that filters be checked and changed daily.
Transoesophageal echocardiography
Transoesophageal echocardiography (TOE) carries a high risk of aerosol generation in an awake or sedated patient because of the risk of coughing. In an anaesthetised, paralysed patient with a cuffed endotracheal tube, the risk is currently unknown, and some regions have determined TOE in intubated patients to be non‐aerosolising.
The American and British Societies of Echocardiography, however, consider TOE to be an aerosol‐generating procedure regardless of intubation status.24,25 Both societies recommend airborne precautions and the most experienced sonographer to perform the TOE.
We recommend that the requirement for TOE be considered on an individual case basis rather than routine utilisation. If necessary, practical interventions to prevent cross‐infection can be considered, including plastic TOE covers, dedicated equipment, a two‐person technique to manipulate probe and acquire images, a plastic barrier over the patient during manipulation, and probe removal and decontamination protocols.
Cardiopulmonary bypass
There is some evidence that SARS‐CoV‐2 RNA has been found in blood.26 It is also possible for the virus to cross the membrane and aerosolise through the gas exit port of the membrane lung during cardiopulmonary bypass, although the risk is extremely low.26 The Australian and New Zealand College of Perfusionists has produced a statement on this matter.27 As per existing guidelines, it is recommended that oxygenators be scavenged similarly to anaesthetic ventilators.28
Inadvertent lung injury during cardiac surgery
Lung trauma may occur at many stages during cardiac surgery. Although there are limited data, we believe that an open pleural space without air leak is relatively low risk for aerosols. However, lung injury may occur on opening the pleura, internal thoracic artery harvest and sternal wire placement. Staff conducting cardiac surgery on patients at high risk of COVID‐19 should employ airborne PPE. Inadvertent lung injury in patients at low risk of COVID‐19 can be managed with standard PPE, with an appropriate protocol in place for visceral pleura breach in times and regions of high community transmission. A suggested approach would be to stop ventilation, pack lung, recommence ventilation at low tidal volume, adopt airborne precautions, temporarily discontinue ventilation, seal air leak, and recommence ventilation.
Trainees and training
Training of junior doctors is always a high priority. Care must be taken in the current environment that this is always done with safety in mind. Appropriate case selection and consideration of the duration of operations are important. Procedures that are high risk of aerosol generation and spread of COVID‐19 are not appropriate for allocation to trainees.
Conclusion
There are a number of procedures that represent a high risk of aerosol generation during cardiothoracic surgery. It is important to follow recommendations to reduce that risk and protect health care workers involved in these procedures.
Box 1 – Key recommendations
General considerations:
- The COVID‐19 pandemic continues to evolve. Patient and staff management principles should be applied in the light of the specific regional and temporal context of the pandemic.
- Patient risk stratification is important and may be guided by national guidelines.8
- Local prevalence may be used to guide decisions around routine pre‐operative testing.
- Most SARS‐CoV‐2 spread is likely to be contact or droplet related. Aerosol spread is likely to be of lower overall frequency but certain procedures, during thoracic surgery especially, present a high risk of aerosol generation.
- Conservative management or considered delay of confirmed or suspected COVID‐19-positive patients may benefit patients and health care workers.
- COVID‐19 theatre planning should consider theatre airflow, contamination zones, theatre personnel present, additional staff roles, personal protective equipment and appropriate staff briefings.
- Experienced clinicians in cardiothoracic anaesthesia and surgery should be involved in the operative care of cardiothoracic surgical COVID‐19 patients.
- Aerosols may be highest around intubation, extubation and open circuits, which are often a part of thoracic anaesthesia. Steps should be taken to minimise required disconnections and/or aerosolisation during these periods.
- The aerosol risk from transoesophageal echocardiography may be amplified by coughing and gagging. It is appropriate to consider the risk–benefit balance for transoesophageal echocardiography and practical interventions to reduce risk.
- Aerosol generation may occur with surgical opening of the lungs and surgical trauma. Predefined procedures to respond to such scenarios should be considered and practised.
- The risk of viral transmission via cardiopulmonary bypass circuit oxygenators is unknown. Scavenging similar to that attached to anaesthetic machines may minimise potential risk.
- Chest drain management should include the use of viral filters on the chest drain exhaust to minimise the risk of aerosolisation of the virus.
Provenance: Not commissioned; externally peer reviewed.
- Joanne F Irons1,2
- Warren Pavey3,4
- Jayme S Bennetts5
- Emily Granger6
- Elli Tutungi7
- Aubrey Almeida7
- 1 Royal Prince Alfred Hospital, Sydney, NSW
- 2 University of Sydney, Sydney, NSW
- 3 Fiona Stanley Hospital, Perth, WA
- 4 University of Notre Dame Australia, Fremantle, WA
- 5 Flinders Medical Centre, Adelaide, SA
- 6 St Vincent's Hospital, Sydney, NSW
- 7 Monash Health, Melbourne, VIC
We thank the members of the Cardiac Thoracic Vascular and Perfusion Special Interest Group executive committee for their review and comments.
No relevant disclosures.
- 1. John Hopkins University and Medicine. Coronavirus Resource Center. https://coronavirus.jhu.edu/ (viewed May 2020).
- 2. World Health Organization. Modes of transmission of virus causing COVID‐19: implications for IPC precaution recommendations: scientific brief, 29 March 2020. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (viewed May 2020)
- 3. Collinson T, Hewitt P, Hugh T et al. Surgery triage: responding to the COVID‐19 pandemic. A rapid review commissioned by RACS. Version 2.0, 5 May 2020. Melbourne: Royal Australasian College of Surgeons, 202. https://umbraco.surgeons.org/media/5254/2020-04-22_racs-triage-of-surgery-web.pdf (viewed May 2020).
- 4. NSW Government Clinical Excellence Commission. Infection prevention and control application of PPE during COVID‐19, version 2.3, August 2020. http://cec.health.nsw.gov.au/__data/assets/pdf_file/0006/590307/Application-of-PPE-in-COVID-19.pdf (viewed Sept 2020).
- 5. Victorian Government Department of Health and Human Services. Coronavirus disease 2019 (COVID‐19): guidance on the use of personal protective equipment (PPE) for health workers. https://www.dhhs.vic.gov.au/personal-protective-equipment-ppe-covid-19 (viewed June 2020).
- 6. National Health and Medical Research Council and Australian Commission on Safety and Quality in Health Care. Australian guidelines for the prevention and control of infection in healthcare. Canberra: NHMRC, ACSQHC, 2019. https://www.nhmrc.gov.au/about-us/publications/australian-guidelines-prevention-and-control-infection-healthcare-2019 (viewed June 2020).
- 7. Ran L, Chen X, Wang Y, et al. Risk factors of healthcare workers with coronavirus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis 2020;. https://doi.org/10.1093/cid/ciaa287. [Epub ahead of print].
- 8. Communicable Diseases Network Australia. Coronavirus disease 2019 (COVID‐19). CDNA national guidelines for public health units. Canberra: CDNA, 2020. https://www1.health.gov.au/internet/main/publishing.nsf/Content/7A8654A8CB144F5FCA2584F8001F91E2/$File/COVID-19-SoNG-v3.8.pdf (viewed Sept 2020).
- 9. Watson J, Whiting PF, Brush JE. Interpreting a covid‐19 test result. BMJ 2020; 369: m1808.
- 10. Arevalo‐Rodriguez I, Buitrago‐Garcia D, Simancas‐Racines D, et al. False‐negative results of initial RT‐PCR assays for COVID‐19: a systematic review. medRxiv 2020; https://doi.org/10.1101/2020.04.16.20066787.
- 11. Zaman S, MacIsaac AI, Jennings GL, et al. Cardiovascular disease and COVID‐19: Australian/New Zealand consensus statement. Med J Aust 2020; 213: 182–187. https://www.mja.com.au/journal/2020/213/4/cardiovascular-disease-and-covid-19-australian-and-new-zealand-consensus
- 12. Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID‐19 infection. EClinicalMedicine 2020; 21: 100331.
- 13. Tran K, Cimon K, Severn M, et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7: e35797.
- 14. Wilson NM, Norton A, Young FP, Collins DW. Airborne transmission of severe acute respiratory syndrome coronavirus‐2 to healthcare workers: a narrative review. Anaesthesia 2020; 75: 1086–1095.
- 15. Australian and New Zealand College of Anaesthetists. ANZCA statement on personal protection equipment during the SARS‐CoV‐2 pandemic (15 May 2020). Melbourne and Wellington: ANZCA, 2020. https://www.anzca.edu.au/documents/pcu_anzca-covid-ppe-statement_20200330.pdf (viewed May 2020).
- 16. Australian Society of Anaesthetists. ASA staff safety for COVID‐19. 11 August 2020. Sydney: ASA, 2020. https://www.asa.org.au/wordpress/wp-content/uploads/News/eNews/covid-19/ASA_staff_protection_background.pdf (viewed Sept 2020).
- 17. Brewster DJ, Chrimes N, Do TB, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID‐19 adult patient group. Med J Aust 2020; 212: 472–481. https://www.mja.com.au/journal/2020/212/10/consensus-statement-safe-airway-society-principles-airway-management-and-0
- 18. Campos JH. Lung isolation techniques. Anesthesiol Clin North Am 2001; 19: 455–474.
- 19. Şentürk M, El Tahan MR, Szegedi LL, et al. Thoracic anesthesia of patients with suspected or confirmed 2019 novel coronavirus infection: preliminary recommendations for airway management by the European Association of Cardiothoracic Anaesthesiology Thoracic Subspecialty Committee. J Cardiothorac Vasc Anesth 2020; 34: 2315–2327.
- 20. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence‐based recommendations for point‐of-care lung ultrasound. Intensive Care Med 2012; 38: 577–591.
- 21. Levy‐Faber D, Malyanker Y, Nir RR, et al. Comparison of VivaSight double‐lumen tube with a conventional double‐lumen tube in adult patients undergoing video‐assisted thoracoscopic surgery. Anaesthesia 2015; 70: 1259–1263.
- 22. Bilkhu R, Viviano A, Saftik I, Billè A. COVID‐19: chest drains with air leak – the silent ‘super spreader’? CTSNet 2020; 2020: https://doi.org/10.25373/ctsnet.12089130.v1 (viewed May).
- 23. Barr J, Internullo E, West D, et al. COVID‐19: safe thoracic surgery. CTSNet 2020; 2020: https://doi.org/10.25373/ctsnet.12200786 (viewed June).
- 24. Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Soc Echocardiogr 2020; 33: 648–653.
- 25. British Society of Echocardiography. Clinical guidance regarding provision of echocardiography during the COVID‐19 pandemic: April 2020. https://bsecho.org/covid19 (viewed May 2020).
- 26. Squiccimarro E, Rociola R, Haumann RG, et al. Extracorporeal oxygenation and COVID‐19 epidemic: is the membrane fail‐safe to cross contamination? ASAIO J 2020; 66: 841–843.
- 27. Baker R, Preovolos A. Is COVID‐19 able to be transmitted across the membranes of our oxygenators in the exhaust gases during CPB or ECMO? Melbourne: Australian and New Zealand College of Perfusionists, 2020. https://anzcp.org/wp-content/uploads/2020/06/ANZCP-Exhaust-Gas-Covid-19-Response-Document-.pdf (viewed June 2020).
- 28. Wahba A, Milojevic M, Boer C, et al. 2019 EACTS/EACTA/EBCP guidelines on cardiopulmonary bypass in adult cardiac surgery. Eur J Cardiothorac Surg 2020; 57: 210–251.





Abstract
Introduction: Coronavirus disease 2019 (COVID‐19) is a contagious disease that is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Health care workers are at risk of infection from aerosolisation of respiratory secretions, droplet and contact spread. There are a number of procedures that represent a high risk of aerosol generation during cardiothoracic surgery. It is important that adequate training, equipment and procedures are in place to reduce that risk.
Recommendations: We provide a number of key recommendations, which reduce the risk of aerosol generation during cardiothoracic surgery and help protect patients and staff. These include general measures such as patient risk stratification, appropriate use of personal protective equipment, consideration to delay surgery in positive patients, and careful attention to theatre planning and preparation. There are also recommended procedural interventions during airway management, transoesophageal echocardiography, cardiopulmonary bypass, chest drain management and specific cardiothoracic surgical procedures. Controversies exist regarding the management of low risk patients undergoing procedures at high risk of aerosol generation, and recommendations for these patients will change depending on the regional prevalence, risk of community transmission and the potential for asymptomatic patients attending for these procedures.
Changes in management as a result of this statement: This statement reflects changes in management based on expert opinion, national guidelines and available evidence. Our knowledge with regard to COVID‐19 continues to evolve and with this, guidance may change and develop. Our colleagues are urged to follow national guidelines and institutional recommendations regarding best practices to protect their patients and themselves.
Endorsed by: Australian and New Zealand Society of Cardiac and Thoracic Surgeons and the Anaesthetic Continuing Education Cardiac Thoracic Vascular and Perfusion Special Interest Group.