On 11 March 2020, the World Health Organization declared coronavirus disease 2019 (COVID‐19) a pandemic. The presence of underlying cardiovascular disease (CVD) confers the highest mortality with COVID‐19. Thus, patients with CVD must be considered a particularly at‐risk population.1,2,3,4,5 Community transmission, patient‐to‐patient transmission and health care worker infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) are overwhelming health services worldwide.4,5,6 High quality cardiac care must minimise risk of viral transmission to patients and health care workers. It should adapt resources in the context of reduced access to hospital beds and personal protective equipment (PPE). This consensus statement reviews and summarises data on SARS‐CoV‐2 infection in pre‐existing CVD and acute cardiovascular manifestations of COVID‐19, and makes recommendations for cardiac service provision during the pandemic.
Development process for the recommendations
A group of CVD experts, drawn from the Cardiac Society of Australia and New Zealand (CSANZ), the Australian and New Zealand Society of Cardiac and Thoracic Surgeons, the National Heart Foundation of Australia and the High Blood Pressure Research Council of Australia, convened in March 2020. Key opinion leaders in cardiology, cardiothoracic surgery and public health with broad geographic representation were consulted. We searched major databases (EMBASE, MEDLINE and PubMed) to identify relevant systematic reviews, randomised controlled trials and clinical case series in English from January 2020 to 25 March 2020. As the majority of studies relating to COVID‐19 and CVD at the time of writing were observational in nature, results must be interpreted with caution. Given data limitations, consensus documents produced by international cardiology societies from December 2019 to March 2020 were reviewed.7,8,9 Experts from key areas (electrophysiology and pacing, interventional cardiology, imaging, cardiothoracic surgery, nursing, hypertension, prevention and rural) generated key recommendations from their respective councils and groups. In addition, social networking platforms (eg, WhatsApp) involving CSANZ board members, cardiology heads of department and key opinion leaders were used to identify relevant resources, guidance documents and protocols. An online living document was shared to facilitate wide input. The full draft underwent peer review by the listed authors as well as external experts in each subspecialty field of cardiology before agreement and acceptance of the final document.
Pre‐existing cardiovascular disease and COVID‐19
Patients with COVID‐19 and pre‐existing CVD are at increased risk of severe disease and death.1,2,3,4,5 A meta‐analysis of eight studies and over 46 000 patients in China reported that hypertension, diabetes and CVD were the most common comorbidities.5 Baseline CVD conferred the highest odds of any comorbidity for developing severe versus mild COVID‐19 (odds ratio [OR], 3.42; 95% CI, 1.88–6.22). Hypertension (OR, 2.36; 95% CI, 1.46–3.83) and respiratory disease (OR, 2.46; 95% CI, 1.76–3.44) also increased the risk of severe COVID‐19,5 while smoking did not.10 Patients with pre‐existing CVD had high case fatality rates; five times that of the overall COVID‐19 infected population (Box 1).4 In Italy, the case fatality rate has been the highest reported worldwide at 12.8%, with a corresponding high prevalence of baseline CVD in fatal cases.11
Pre‐existing CVD confers an increased risk of death with COVID‐19; health services and patients should therefore take additional precautions.
Angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers
As SARS‐CoV‐2 enters cells by binding to human angiotensin‐converting enzyme 2 (ACE2) receptors found in the lungs and heart,12 activation of the renin–angiotensin system may contribute to the increased susceptibility to infection in patients with pre‐existing CVD.13 It has been suggested that angiotensin‐converting enzyme inhibitors (ACE‐I) and angiotensin receptor blockers (ARBs) may increase the risk of SARS‐CoV‐2 infection or worsen the outcome,14 as treatment with ACE‐I or ARBs can increase the expression and activity of ACE2 in some animal models.15 However, there is no clinical evidence substantiating an adverse effect of ACE‐I or ARBs on COVID‐19 outcomes. Conversely, there is evidence for protective effects from mouse models,16 and recombinant ACE2 and the ARB losartan are currently being tested in the United States as potential COVID‐19 therapies.17 An Italian case–control study revealed that while ACE‐I or ARB use was more frequent among COVID‐19 patients owing to higher CVD prevalence, they did not influence COVID‐19 severity.18
Given the well established beneficial effects of ACE‐I and ARBs, we, along with numerous national and international societies, strongly recommend that these medications be continued.19,20,21
Acute cardiac injury and COVID‐19
Acute cardiac injury in COVID‐19 manifests as left ventricular dysfunction, heart failure, ventricular arrhythmias, electrocardiogram (ECG) changes, and elevated B‐type natriuretic peptide and troponin levels.2,22,23,24 In the first 41 confirmed Chinese COVID‐19 cases, acute cardiac injury defined as elevated cardiac biomarkers with ECG changes and left ventricular dysfunction was seen in 12% of patients.2 A later study found acute cardiac injury in 19.7% of participants,24 while a US study of 21 intensive care unit (ICU) patients described cardiomyopathy in 33%.22 Acute cardiac injury was independently associated with mortality in hospitalised patients with COVID‐19 in China.24
Pathophysiological theories for cardiac injury include direct infection of the myocardium with SARS‐CoV‐2, myocardial inflammation, Takotsubo syndrome and overwhelming multi‐organ sepsis. While direct viral spread via ACE2 receptors in the myocardium has been postulated, a histopathological study of COVID‐19‐associated cardiomyopathy did not find direct SARS‐CoV‐2 infection.25 Myocardial inflammatory infiltrates were instead seen.26 For patients with left ventricular dysfunction, ACE‐I, ARBs and β‐blockers are indicated as the proposed pathophysiology of renin–angiotensin system imbalance with COVID‐19 points to their potential therapeutic roles. However, much more study is needed to define the underlying pathophysiology and optimal treatment.
Elevated troponin levels and myocardial infarction
Troponin and other cardiac enzymes are commonly elevated in COVID‐19.2,3,5,22,26 Troponin elevation is a prognostic marker and may reflect myocarditis or myocardial infarction (MI).27 The diagnostic implications are unclear as it can be associated with non‐coronary conditions including acute respiratory infections28 and type 2 MI.29 Myocardial injury in COVID‐19 patients can manifest with ST elevation in the absence of obstructive coronary artery disease. Many pathophysiological mechanisms have been proposed for COVID‐19‐related myocardial injury, including myocarditis, microvascular injury or obstruction, endothelial dysfunction and acute plaque rupture. In addition, a prothrombotic state has been commonly described with prominent elevations of D‐dimer and fibrin/fibrinogen degradation products.30,31,32
In the setting of acute COVID‐19 illness and suspected non‐ST elevation (NSTEMI), patients who are haemodynamically stable without ongoing ischaemia may be best managed conservatively, with invasive procedures deferred until after recovery.
Cardiovascular implications of novel therapies
Numerous clinical trials assessing treatment for COVID‐19 are being conducted. Chloroquine, hydroxychloroquine, azithromycin and ritonavir–lopinavir are under investigation, alone or in combination. These medications can cause cardiac toxicity, specifically QTc prolongation and torsades de pointes, especially in patients with hepatic or renal dysfunction.33
Off‐label prescribing of hydroxychloroquine has been reported and health professionals should be alert to cardiac toxicity in the community.
Recommendations for cardiovascular health care services
Safety is of paramount importance to limit COVID‐19 exposure in high risk cardiology patients and our workforce. All patients need to be risk assessed for COVID‐19 status to guide appropriate infection control measures (Box 2). All health services need to review elective procedures in order to increase hospital capacity and conserve valuable PPE. Alternative health care for patients at risk of COVID‐19 that avoids exposure within the hospital system requires multidisciplinary assessment. Cardiac procedures that require long stays in hospital or the ICU should be carefully considered, owing to their impact on bed availability. An adaptable threshold for acute cardiology admissions and cardiac monitoring is needed. Stable angina, troponin‐negative chest pain, non‐life‐threatening arrhythmias or cardiac diagnoses without clinical instability may be managed in an outpatient setting. Rapid discharge strategies should be instituted, including same‐day discharge for elective percutaneous coronary intervention (PCI), and next‐day discharge for NSTEMI following revascularisation. As some elective procedures or hospital admissions cannot be safely postponed, nuanced clinical judgement is required.
Key considerations in the management of acute MI and coronary angiography
A critical concern during the COVID‐19 pandemic is use of the cardiac catheterisation laboratory (Box 3). Bringing a COVID‐19‐positive patient to the cardiac catheterisation laboratory exposes staff to the risk of infection, and prevents post‐procedure laboratory use pending a terminal clean. Delays are to be expected with primary PCI to allow for COVID‐19 assessment and infection control measures. ST elevation MI (STEMI) protocols from China and the US during the COVID‐19 pandemic have been published.23,29 The Sichuan Provincial People's Hospital proposed fibrinolytic therapy for all STEMIs, with suspension of their primary PCI service.29 This lysis protocol relied on rapid nucleic acid testing, which may be limited by individual hospital availability and policy. The US Cardiology Society recommended primary PCI continuation with appropriate PPE, and lysis for select cases.23 In Australia, each health care service will be different, but it is important that a local protocol is developed and adapted (Box 4), with CSANZ guidance available.34 Training in PPE, sourcing fibrinolytic medications and updating lysis protocols are critical. As COVID‐19 is associated with STEMI mimickers (ST elevation without obstructive coronary artery disease due to microvascular thrombosis or myocarditis), use of lysis may confer risk without benefit in some cases, exacerbated by COVID‐associated coagulation abnormalities.17,30 Bedside echocardiography to ascertain regional wall abnormalities and computed tomography coronary angiography to limit cardiac catheterisation laboratory staff exposure could all be considered.
Reliance on the presence of an elevated troponin level to indicate NSTEMI in COVID‐19 patients will be misleading. Greater emphasis should be given to high risk clinical features such as recurrent chest pain, dynamic ischaemic ECG changes, heart failure, haemodynamic instability, major arrhythmias and regional wall motion abnormalities. It is reasonable to defer invasive investigations in stable COVID‐19‐positive patients without high risk features.
Regional and remote cardiovascular services
In Australia, established pre‐hospital lysis programs currently exist, with cardiologist‐led 24/7 ECG‐reading service and pre‐hospital/small hospital lysis for STEMI where PCI access is limited. Patients are then transferred to a PCI‐capable hospital. These transfers will require additional screening for COVID‐19. A greater level of cardiologist‐led telehealth support for regional and rural centres will be needed. Centralised ECG‐reading services are well placed to coordinate transfer logistics with linked calls between cardiologists and state retrieval, emergency and ICU consultants, balancing patient needs with staff safety and resource utilisation. It is important to continue to provide STEMI services for non‐COVID‐19 rural and regional patients already at a disadvantage in terms of cardiovascular outcomes, while balancing the enormous resourcing demands that COVID‐19 places on health care systems.
Cardiothoracic surgery considerations
On 25 March 2020, the Australian government suspended non‐urgent surgery (https://www.pm.gov.au/media/elective-surgery#:~:text=The%20National%20Cabinet%20is%20acting,will%20continue%20until%20further%20notice). This suspension was lifted on 27 April 2020, with non‐urgent surgery allowed, at a reduced capacity (https://www.health.gov.au/news/australian-health-protection-principal-committee-ahppc-statement-on-restoration-of-elective-surgery-and-hospital-activity). However patients with symptomatic disease will continue to present to hospital and require inpatient cardiac surgery. Cardiac surgical cases are likely to take longer during the COVID‐19 pandemic owing to infection control measures, and access to the ICU may at times be limited. The multidisciplinary heart team will need to consider and adjust thresholds for management of coronary artery disease in the event of increased hospital COVID‐19 case load. The same applies to surgical aortic valve replacement or transcatheter aortic valve implantation for severe symptomatic aortic valve stenosis. The inherent risk of the untreated cardiovascular condition will need to be weighed against the risk of nosocomial infection during hospitalisation and the implications for ventilator use, bed stay and recovery time. Establishing or re‐establishing cardiac surgical ICU programs with outcome measures equivalent to those of the general ICU35 could be considered in the future if needed, to free up precious general ICU resources.
Key considerations in imaging and stress testing
During the COVID‐19 pandemic, elective cardiac investigations will need to be prioritised, based on short term management change versus risk of deferment until the pandemic passes. Certain cardiac investigations such as stress testing and transoesophageal echocardiography pose significant viral transmission risk. Exercise stress testing can result in droplet spread, whereas pharmacological testing that obviates the need for exercise or a technician in close proximity may be relatively safer.36 Transoesophageal echocardiography involves instrumentation of the oropharynx, known to harbour the virus with high risk of aerosol transmission,37 and should be undertaken only if transthoracic echocardiography cannot be performed, or after exclusion of COVID‐19. If transesophageal echocardiography is performed in a patient with COVID‐19, it should be occur in a negative pressure room or with patient intubation with appropriate PPE. General considerations for transthoracic and transoesophageal echocardiography for patients with suspected or confirmed COVID‐19 are summarised in Box 5.
Acute cardiac care and cardiopulmonary resuscitation
In COVID‐19 cases with concomitant heart failure and hypoxaemia, non‐invasive ventilation such as continuous positive airway pressure and bilevel positive airway pressure is aerosol generating and not recommended.37,38 High flow nasal oxygen can be used within single rooms or shared COVID‐19 ward spaces with strict attention to staff safety and the lowest flow necessary to maintain oxygen saturation ≥ 92%. Cardiopulmonary resuscitation is another important consideration, currently under review by the Australian National COVID‐19 Taskforce. Defibrillation is not aerosol generating but all other resuscitative procedures are, and PPE is therefore recommended.39 Ways to reduce COVID‐19 transmission during cardiopulmonary resuscitation include covering the patient's mouth and nose with a mask, towel or plastic cover, and using mechanical compression devices. Discussion of goals of care remains important in both the inpatient and community setting. Management of hypoxaemia and cardiac arrest in patients with suspected or confirmed COVID‐19 is summarised in Box 6.
Key considerations in electrophysiology and pacing services
The COVID‐19 pandemic poses particular challenges in cardiac arrhythmia management, as patients require outpatient clinic review, ambulatory monitoring, electrophysiological interventions, implantation and follow‐up of cardiac implanted electronic devices (Box 7). A team‐based approach is advised, with teleconferences at weekly intervals to ensure maintenance of appropriateness criteria, urgency, and alignment of practices with the local outbreak response.
Considerations for outpatient cardiology care and use of telehealth
Strategies to minimise COVID‐19 exposure in cardiovascular outpatient clinics must be adopted, including government recommendations for physical distancing. Serious consideration should be given to using telehealth for all outpatient consultations. For in‐person consultations, verbal and temperature screening and social distancing are required. Administrative teams should be supported in their ability to maintain physical distancing to reduce their own exposure. Nurse‐led clinics and cardiac rehabilitation programs will need to adapt by using telehealth or digital health platforms. Patients can be monitored and supported at home remotely, ensuring adequate medication supply, using a set of scales and blood pressure machines to titrate medications. Online support can enable patients to continue cardiac rehabilitation during home isolation.
Considerations for primary care and cardiovascular disease in the community
The importance of maintaining contact with patients by primary care physicians is highlighted by trends showing an unintended consequence of COVID‐19 is less frequent primary care and delayed hospital presentations. The National Heart Foundation has released public health messages emphasising the importance of medication continuation and staying connected to treating doctors, and that it is both safe and beneficial to present to hospital if required.40
Health care workers
There is a considerable risk of SARS‐CoV‐2 infection among health care workers.4,6 Health care services need to ensure adequate protection with appropriate PPE in the care of COVID‐19 patients. This includes fitted respirator masks (N95, FFP2 or equivalent) for any aerosol‐generating procedures, and training in correct PPE use. Services may need to adapt to health care worker shortages and leave due to illness or quarantine. Cardiology trainees may also be affected by re‐allocation within the hospital. The decision to move to a weekly rotation of staggered cardiology teams (relevant for clinicians, surgeons, sonographers and STEMI on‐call teams) may limit infection of all staff if case numbers rise. Links to relevant documents and important websites are provided in the Supporting Information.
Conclusion
The COVID‐19 pandemic is constantly evolving, and we must both prepare for and adapt to this global health care crisis. Cardiology services and providers need to develop and refine COVID‐19 strategies according to the level of community infection and in line with both local hospital and governmental policy.
Box 1 – Case fatality rates of patients with COVID‐19 with selected comorbidities*
|
Condition |
Case fatality rate |
||||||||||||||
|
|
|||||||||||||||
|
Cardiovascular disease |
10.5% |
||||||||||||||
|
Diabetes |
7.3% |
||||||||||||||
|
Chronic respiratory disease |
6.3% |
||||||||||||||
|
Hypertension |
6.0% |
||||||||||||||
|
Cancer |
5.6% |
||||||||||||||
|
No comorbidities |
0.9% |
||||||||||||||
|
|
|||||||||||||||
|
* Data from 44 672 confirmed COVID‐19 cases from mainland China with an overall case fatality rate of 2.3% (1023 deaths).4 |
|||||||||||||||
Box 2 – Assessment of patient risk for COVID‐19
Clinical and radiological risk factors:*- fever > 37.5°C (patients may have no or low grade fever on presentation)
- cough, shortness of breath or sore throat
- any influenza‐like symptoms in a health care worker
- contact with a confirmed COVID‐19 contact in the past 14 days
- arrival from overseas in the past 14 days
- lymphocyte count < 1.5 x 109/L
- bi‐basal ground glass appearance (computed tomography) or bi‐basal pneumonia (chest x‐ray)
* Note: These risk factors may change; check the Australian Government Department of Health website at https://www.health.gov.au/news/health-alerts/novel-coronavirus-2019-ncov-health-alert for updates.
Box 3 – General considerations for catheterisation laboratory use during the COVID‐19 pandemic
- • Determine the patient's COVID‐19 status (see Box 2):
- ▶ when available, consider rapid point‐of-care testing; if unable to obtain history (eg, intubated patient), consider the patient to be at risk
- • For all confirmed or suspected COVID‐19 cases:
- ▶ patients: surgical/medical mask if not intubated
- ▶ all catheterisation laboratory staff: personal protective equipment including aerosol protection (N95 mask), given risk of emergent intubation/cardiopulmonary resuscitation/vomiting in ST elevation myocardial infarction (aerosol‐generating procedures)
- • Patients approaching or requiring intubation should receive this before transfer to the cardiac catheterisation laboratory, as intubation/suction/active cardiopulmonary resuscitation all increase aerosolisation of respiratory secretions
- • Designate “dirty” COVID‐19 catheterisation laboratories within each institution that are cleared of non‐essential equipment and stock to facilitate cleaning. Consider dedicated laboratory stock for COVID‐19 patients
- • Limit the number of staff in the cardiac catheterisation laboratory to essential personnel (eg, cardiologist, scrub assistant, scout nurse)
- • Minimise or abolish staff movements in and out of the laboratory during the case
- • Institute a dedicated “clean” nurse role outside the laboratory to allow for passing equipment and medication, coordinate destination teams for transfer, and facilitate correct use of personal protective equipment
- • A terminal clean following the procedure is required, with potential for delays in subsequent cases
Box 4 – General principles to consider for management of ST elevation myocardial infarction (STEMI) during the COVID‐19 pandemic
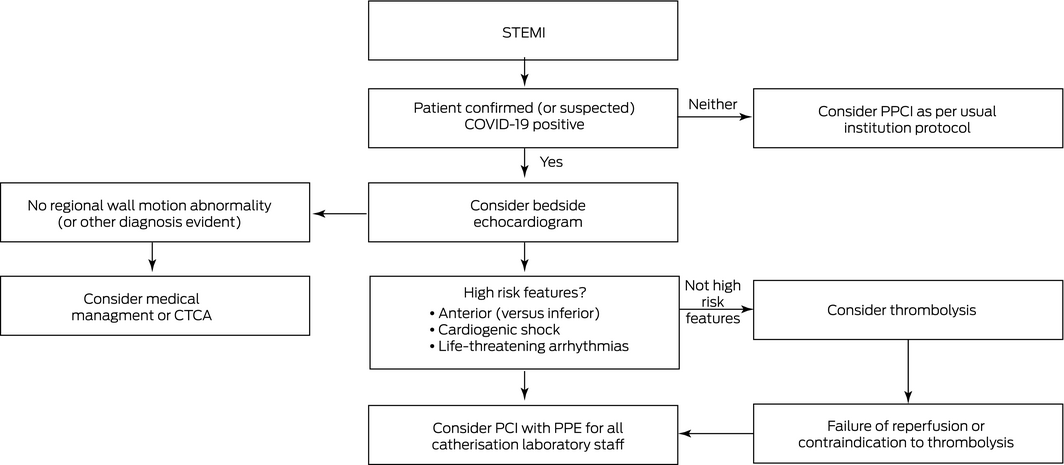
CTCA = computed tomography coronary angiography; PCI = percutaneous coronary intervention; PPCI = primary PCI; PPE = personal protective equipment. Note: At any stage in this pathway either PPCI or thrombolysis could be considered.
Box 5 – General considerations for transthoracic and transoesophageal echocardiography for patients with suspected or confirmed COVID‐19
- Personal protective equipment for health care providers and assistants performing the test
- Shorten study duration to reduce face‐to‐face contact; eg, limiting transthoracic echocardiography to 15 minutes
- Dedicated COVID‐19 machine and equipment
- Perform test in patient's room; do not bring patient to the cardiology department
- Plastic disposable covers for the machine and equipment, removed inside the room on completion, followed by complete clean of equipment with alcohol
- Consider hand‐held or portable echocardiography if available
- Transoesophageal echocardiography has high risk for respiratory transmission and should be performed only if result will change treatment, in negative pressure room or designated theatre space
- Exercise electrocardiography and exercise stress echocardiography have high respiratory transmission risk and suspension of these services should be carefully considered
Box 6 – Management of hypoxaemia and cardiac arrest in patients with suspected or confirmed COVID‐19
- • Continuous positive airway pressure and bilevel positive airway pressure are aerosol generating and should be avoided
- • High flow nasal oxygen can be used in single rooms or shared COVID‐19 ward spaces with appropriate staff protection
- • For resuscitation:
- ▶ cover the patient's mouth and nose (mask, towel or other cover)
- ▶ early defibrillation is life saving and not considered aerosol generating
- ▶ chest compressions, assisted ventilation and advanced airway manoeuvres are considered aerosol generating (weak level of evidence)39 and personal protective equipment should be worn
- ▶ use mechanical compression devices if available
- • Consider and document advance care directives
Box 7 – General considerations for electrophysiology and pacing during the COVID‐19 pandemic
- • For cardiac implanted electronic device follow‐up:
- ▶ avoid in‐person clinics, hospitals and office visits
- ▶ use remote monitoring/telehealth
- ▶ for major problems (eg, lead/battery or device therapies in defibrillator patients), consider risks of delayed visit versus risk of COVID‐19 exposure
- • Requests for urgent cardiac implanted electronic device interrogation by wards and emergency departments:
- ▶ use remote monitoring and/or manual transmissions
- ▶ face-to‐face interrogation requires use of personal protective equipment and minimal number of technicians with wireless technology if possible
- • Defer elective electrophysiology procedures for 1–3 months until personal protective equipment stocks are sufficient
- • Urgent procedures to be continued: pacemaker for atrioventricular block and asystolic pauses; generator change for pacing dependent patients; secondary prevention defibrillators; catheter ablation in selective patients with ventricular tachycardia storm; lead extraction as determined by specialist centres
- • Avoid ambulatory monitoring due to low yield; consider mail‐out mobile electrocardiogram monitors
Provenance: Not commissioned; externally peer reviewed.
- Sarah Zaman1,2
- Andrew I MacIsaac3
- Garry LR Jennings4,5
- Markus P Schlaich5,6
- Sally C Inglis7
- Ruth Arnold8
- Saurabh Kumar9,10
- Liza Thomas4,9
- Sudhir Wahi11
- Sidney Lo12
- Carolyn Naismith13
- Stephen J Duffy14,15
- Stephen J Nicholls1,2
- Andrew Newcomb16
- Aubrey A Almeida17,18
- Selwyn Wong19
- Mayanna Lund19
- Derek P Chew20
- Leonard Kritharides21,22
- Clara K Chow9,10
- Ravinay Bhindi23
- 1 MonashHeart, Monash Health, Melbourne, VIC
- 2 Monash Cardiovascular Research Centre, Monash University, Melbourne, VIC
- 3 St Vincent's Hospital, Melbourne, VIC
- 4 University of Sydney, Sydney, NSW
- 5 Baker Heart and Diabetes Institute, Melbourne, VIC
- 6 Dobney Hypertension Centre, University of Western Australia, Perth, WA
- 7 University of Technology, Sydney, NSW
- 8 Orange Health Service, Orange, NSW
- 9 Westmead Hospital, Sydney, NSW
- 10 Westmead Applied Research Centre, University of Sydney, Sydney, NSW
- 11 Princess Alexandra Hospital, Brisbane, QLD
- 12 Liverpool Hospital, Sydney, NSW
- 13 Austin Hospital, Melbourne, VIC
- 14 Alfred Hospital, Melbourne, VIC
- 15 Centre of Cardiovascular Research and Education in Therapeutics, Monash University, Melbourne, VIC
- 16 St Vincent's Clinical School, Melbourne, VIC
- 17 Cardiac Sciences Clinical Institute, Epworth Richmond Hospital, Melbourne, VIC
- 18 Monash Health, Melbourne, VIC
- 19 Middlemore Hospital, Auckland, New Zealand
- 20 Flinders University, Adelaide, SA
- 21 Concord Hospital, Sydney, NSW
- 22 ANZAC Research Institute, Sydney, NSW
- 23 Royal North Shore Hospital, Sydney, NSW
Sarah Zaman and Ravinay Bhindi have been supported by fellowships from the National Heart Foundation of Australia. The funding body had no role in the consensus statement itself.
Sarah Zaman reports research grants from Abbott Australia and speaking honorarium from AstraZeneca outside the submitted work. Stephen Nicholls received research support from AstraZeneca, Amgen, Anthera, CSL Behring, Cerenis, Eli Lilly, Esperion, Resverlogix, Novartis, InfraReDx and Sanofi‐Regeneron, and is a consultant for Amgen, Akcea, AstraZeneca, Boehringer Ingelheim, CSL Behring, Eli Lilly, Esperion, Kowa, Merck, Takeda, Pfizer, Sanofi‐Regeneron and Novo Nordisk; these interests are all outside the submitted work.
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395: 507–513.
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506.
- 3. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020; 323: 1061–1069.
- 4. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–1242.
- 5. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID‐19) infection: a systematic review and meta‐analysis. Int J Infect Dis 2020; 94: 91–95.
- 6. Livingston E, Bucher K. Coronavirus disease 2019 (COVID‐19) in Italy. JAMA 2020; 323: 1335.
- 7. European Society of Cardiology. COVID‐19 and Cardiology. https://www.escardio.org/Education/COVID-19-and-Cardiology?hit=topbanner (viewed June 2020).
- 8. American College of Cardiology. ACC's COVID‐19 hub. https://www.acc.org/latest-in-cardiology/features/accs-coronavirus-disease-2019-covid-19-hub#sort=%40fcommonsortdate90022%20descending (viewed June 2020).
- 9. British Cardiovascular Society. COVID‐19 clinicians resource hub. May 2020. https://www.britishcardiovascularsociety.org/resources/covid-19-clinicians-hub (viewed June 2020).
- 10. Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID‐19). Eur J Intern Med 2020; 75: 107–108.
- 11. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA 2020; 323: 1775–1776.
- 12. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020; 181: 271–280.
- 13. Tikellis C, Thomas MC. Angiotensin‐converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept 2012; 2012: 256294.
- 14. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med 2020; 8: e21.
- 15. Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation 2005; 111: 2605–2610.
- 16. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med 2005; 11: 875–879.
- 17. Zhang H, Penninger JM, Li Y, et al. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020; 46: 586–590.
- 18. Mancia G, Rea F, Ludergnani M, et al. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med 2020; 382: 2431–2440.
- 19. High Blood Pressure Research Council of Australia. A statement on COVID‐19 and blood pressure medication (ACE‐inhibitors/ARBs). 17 March 2020. https://www.hbprca.com.au/wp-content/uploads/2020/03/HBPRCA-Statement-on-COVID-19-and-BP-medication-17.03.20.pdf (viewed Mar 2020).
- 20. European Society of Cardiology. Position statement of the ESC Council on Hypertension on ACE‐inhibitors and angiotensin receptor blockers. 12 March 2020. https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang (viewed Mar 2020).
- 21. International Society of Hypertension. A statement from the International Society of Hypertension on COVID‐19. https://ish-world.com/news/a/A-statement-from-the-International-Society-of-Hypertension-on-COVID-19/. (viewed Mar 2020).
- 22. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington State. JAMA 2020; 323: 1612–1614.
- 23. Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID‐19 pandemic. J Am Coll Cardiol 2020; 75: 2352–2371.
- 24. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020; 5: 802‐810.
- 25. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8: 420–422.
- 26. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062.
- 27. Lippi G, Lavie CJ, Sanchis‐Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID‐19): evidence from a meta‐analysis. Prog Cardiovasc Dis 2020; https://doi.org/10.1016/j.pcad.2020.03.001 [Epub ahead of print].
- 28. Rivara MB, Bajwa EK, Januzzi JL, et al. Prognostic significance of elevated cardiac troponin‐T levels in acute respiratory distress syndrome patients. PLoS One 2012; 7: e40515.
- 29. Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID‐19: the protocols from Sichuan Provincial People's Hospital. Intensive Care Med 2020; 46: 1111‐1113.
- 30. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol 2020; 75: 2950–2973.
- 31. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood 2020; 135: 2033–2040.
- 32. Klok FA, Kruip M, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: an updated analysis. Thromb Res 2020; 191: 148–150.
- 33. Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: a pilot observational study. Travel Med Infect Dis 2020; 34: 101663.
- 34. Cardiac. Society of Australia and New Zealand. COVID‐19 resources. https://www.csanz.edu.au/covid-19/ (viewed June 2020).
- 35. Lee LS, Clark AJ, Namburi N, et al. The presence of a dedicated cardiac surgical intensive care service impacts clinical outcomes in adult cardiac surgery patients. J Card Surg 2020; 35: 787–793.
- 36. Skali H, Murthy VL, Al‐Mallah MH, et al. Guidance and best practices for nuclear cardiology laboratories during the coronavirus disease 2019 (COVID‐19) pandemic: an information statement from ASNC and SNMMI. J Nucl Cardiol 2020; 27: 1022‐1029.
- 37. Brewster DJ, Chrimes N, Do TBT, et al. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID‐19 adult patient group. Med J Aust 2020; 212: 472–481. https://www.mja.com.au/journal/2020/212/10/consensus-statement-safe-airway-society-principles-airway-management-and-0.
- 38. Cheung JC, Ho LT, Cheng JV, et al. Staff safety during emergency airway management for COVID‐19 in Hong Kong. Lancet Respir Med 2020; 8: e19.
- 39. Craig S, Cubitt M, Jaison A, et al. Management of adult cardiac arrest in the COVID‐19 era: interim guidelines from the Australasian College for Emergency Medicine. Med J Aust 2020; 213: 126–133.
- 40. National Heart Foundation. COVID‐19 and heart disease. https://campaigns.heartfoundation.org.au/covid-19/ (viewed May 2020).





Abstract
Introduction: The coronavirus 2019 disease (COVID‐19) pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Pre‐existing cardiovascular disease (CVD) increases the morbidity and mortality of COVID‐19, and COVID‐19 itself causes serious cardiac sequelae. Strategies to minimise the risk of viral transmission to health care workers and uninfected cardiac patients while prioritising high quality cardiac care are urgently needed. We conducted a rapid literature appraisal and review of key documents identified by the Cardiac Society of Australia and New Zealand Board and Council members, the Australian and New Zealand Society of Cardiac and Thoracic Surgeons, and key cardiology, surgical and public health opinion leaders.
Main recommendations: Common acute cardiac manifestations of COVID‐19 include left ventricular dysfunction, heart failure, arrhythmias and acute coronary syndromes. The presence of underlying CVD confers a five‐ to tenfold higher case fatality rate with COVID‐19 disease. Special precautions are needed to avoid viral transmission to this population at risk. Adaptive health care delivery models and resource allocation are required throughout the health care system to address this need.
Changes in management as a result of this statement: Cardiovascular health services and cardiovascular health care providers need to recognise the increased risk of COVID‐19 among CVD patients, upskill in the management of COVID‐19 cardiac manifestations, and reorganise and innovate in service delivery models to meet demands. This consensus statement, endorsed by the Cardiac Society of Australia and New Zealand, the Australian and New Zealand Society of Cardiac and Thoracic Surgeons, the National Heart Foundation of Australia and the High Blood Pressure Research Council of Australia summarises important issues and proposes practical approaches to cardiovascular health care delivery to patients with and without SARS‐CoV‐2 infection.