To the Editor: The initial months of the coronavirus disease 2019 (COVID‐19) pandemic have led to an unprecedented response from the global medical research community.1 Simultaneously, there have been concerns about the rapid publication of misleading, biased studies.2 We systematically evaluated the early global research response to COVID‐19 by characterising the methodological quality of registered COVID‐19 studies. We also compared the research response with previous respiratory viral epidemics: the severe acute respiratory syndrome (SARS), the Middle East respiratory syndrome (MERS) and the influenza A(H1N1)pdm09 virus pandemic.
We reviewed COVID‐19 studies registered from 1 January to 6 May 2020 in five international clinical trial registries:
- Clinicaltrials.gov3 (https://clinicaltrials.gov);
- the International Clinical Trial Registration Platform4 (https://apps.who.int/trialsearch);
- the European Union Clinical Trials Register5 (www.clinicaltrialsregister.eu);
- the International Standardised Randomised Controlled Trial Number6 (www.isrctn.com); and
- the Australia New Zealand Clinical Trials Register7 (www.anzctr.org.au).
The available registries were searched for studies of SARS, MERS and pandemic H1N1/09 virus registered within 6 months, beginning from the month after these epidemics were first detected.
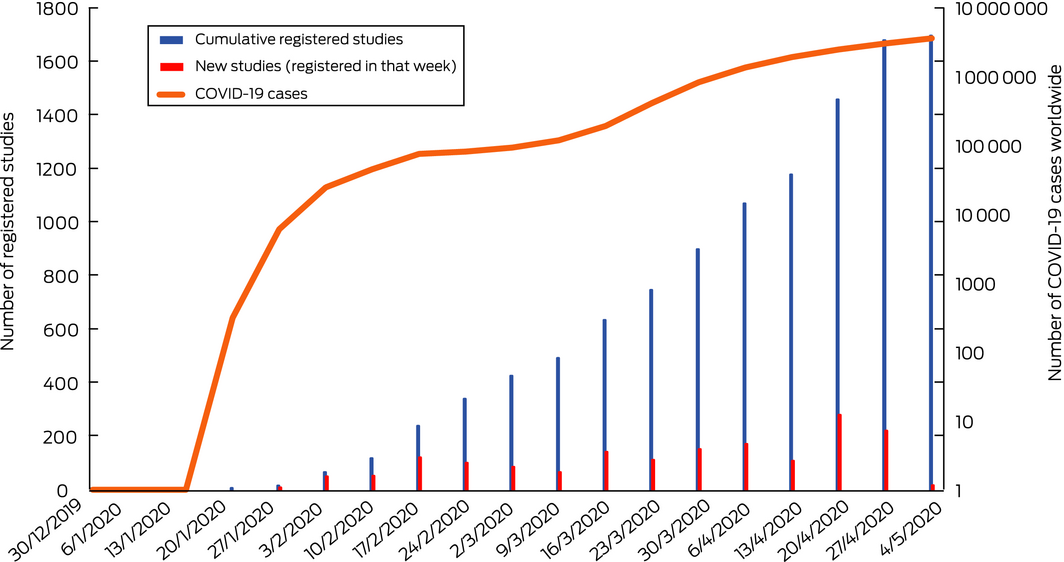
We identified 1694 registered COVID‐19 studies, of which 698 (41%) were randomised controlled trials (RCTs) (Supporting information). Duplicate studies were removed. The growth in the number of registered studies paralleled the rise in confirmed global cases (Box). Of the registered studies, 785 (46%) are currently recruiting participants, 842 (50%) have not commenced recruitment, ten (0.6%) were completed studies and 53 (3%) were withdrawn or suspended.
Most RCTs evaluated interventions for infected subjects (661, 94%), while 37 RCTs (5%) evaluated prophylactic therapies. There were 423 studies (61%) that evaluated drugs, including hydroxychloroquine (122, 17%), lopinavir/ritonavir (36, 5%) and chloroquine (31, 4%). Other interventions included traditional Chinese medicines (84, 12%), biological agents (60, 9%), and vaccines (14, 2%).
Among RCTs, 144 (21%) reported the use of allocation concealment and 253 (36%) reported blinding of the patient, the investigator, the clinician or the outcome assessor. Placebo control was used in 184 RCTs (26%), while 514 (73%) used standard care or active control arms. The presence of a data safety monitoring committee was reported by the majority of RCTs (427, 62%). Only 35 RCTs (5%) reported both measures of internal validity — allocation concealment and blinding.
Six months after the declaration of the SARS and MERS epidemics, there were no registered studies. Comparatively, there were 99 registered studies, of which 71 were RCTs, in the 6 months after the onset of the pandemic H1N1/09 virus in 2009.
The global research response to COVID‐19 has been substantially larger than that observed with previous epidemics and pandemics. The potential drivers of this include the absence of proven therapies,3 ease of transmissibility,4 rapidity of global spread, and high hospitalisation and mortality rate5 coupled with greater pandemic preparedness and ease of greater global collaboration. It is concerning that only a minority of trials adhered to established markers of internal validity, such as blinding, allocation concealment, placebo where applicable, and a data safety monitoring committee presence.
The high discontinuation rate of trials within 5 months into the pandemic could be due to data from case series and observational studies indicating lack of benefit or even harm with the interventions being tested in RCTs, loss of equipoise, or control of the pandemic resulting in fewer eligible patients for enrolment.
The trade‐off for the rapid expansion of COVID‐19 research has been the suspension of non‐COVID‐19 research in several jurisdictions, and a substantive shift by granting bodies to prioritise COVID‐19 research funding away from non‐COVID‐19 research applications.6,7
While the global research response to COVID‐19 has been rapid and substantial, due to methodological insufficiencies, many studies of interventions may not lead to high quality evidence to guide treatment of COVID‐19. Resulting publications from these studies and reasons for discontinuation of studies would be of interest for future investigation. There was significant duplication with multiple trials of several interventions. The impact on non‐COVID‐19 research has been substantial.
The unedited version of this article was published as a preprint on mja.com.au on 30 June 2020.
- 1. COVID‐19 Clinical Research Coalition. Global coalition to accelerate COVID‐19 clinical research in resource‐limited settings. Lancet 2020; 395: 1322–1325.
- 2. Lee AY, Lin MW. Rapid publishing in the era of coronavirus disease 2019 (COVID‐19). Med J Aust 2020; 212: 535–535. https://www.mja.com.au/journal/2020/212/11/rapid-publishing-era-coronavirus-disease-2019-covid-19
- 3. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA 2020; 323: 1824–1836.
- 4. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. N Engl J Med 2020; 382: 1199–1207.
- 5. Guan W, Ni Z, Hu Y, Liang W, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720.
- 6. Thornton J. Clinical trials suspended in UK to prioritise COVID‐19 studies and free up staff. BMJ 2020; 368: m1172.
- 7. Ledford H. Coronavirus shuts down trials of drugs for multiple other diseases. Nature 2020; 580: 15–16.





No relevant disclosures.