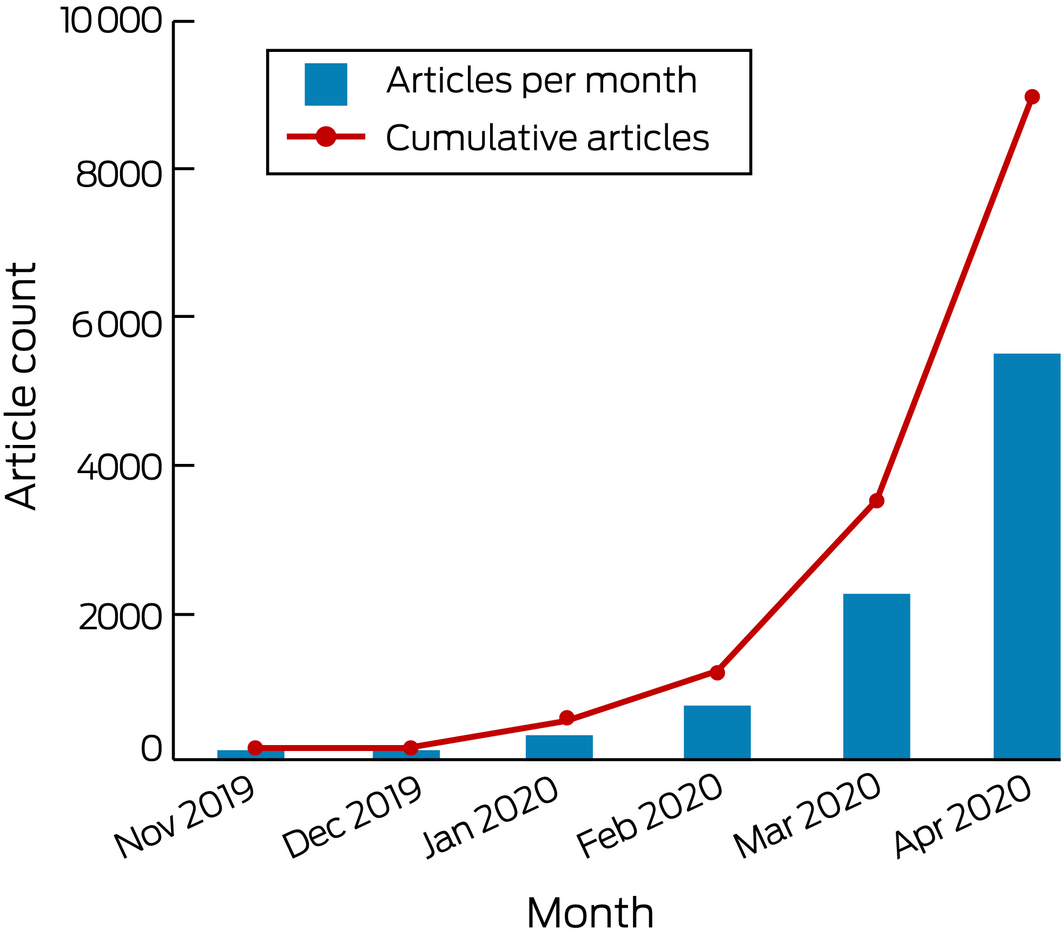
To the Editor: The advent of coronavirus disease 2019 (COVID‐19) has generated an unparalleled level of interest from the medical and non‐medical community. As clinician‐scientists, we watch in astonishment at the exponential growth of academic publications in journals. In January 2020, PubMed saw a sharp rise in the number of publications related to COVID‐19, which continues to grow (Box).
We could not help but wonder if this has generated a race to publish. Of course, publishing is crucial to help confront one of the most devastating global health issues of the century. However, it is well recognised that external pressures to publish can muddle the intrinsic pursuit for scientific curiosity and excellence,1 and COVID‐19 has certainly provided the incentive for many clinicians and scientists alike to seek rapid publication. This may, unfortunately, fuel competition in the research/publishing field, which was exemplified by the concerning lack of research collaborations when humans were faced with natural disasters,2 including the 2003 severe acute respiratory syndrome coronavirus (SARS‐CoV) outbreak.3
The urgent nature of this situation means a number of preliminary studies and publications on COVID‐19 are fast‐tracked through the peer review process — or not at all — in the hope of rapidly publicising important findings, opinions and experiences. However, hastily penned observations may mislead and do more harm than good. A recent non‐peer‐reviewed publication on a preprint server likening SARS‐CoV‐2 structurally to the human immunodeficiency virus (HIV) was quickly retracted after the scientific community highlighted serious flaws in the study.4 Furthermore, a preliminary study5 supporting the use of hydroxychloroquine as a COVID‐19 treatment prompted a flurry of off‐label use and media attention. The study was later criticised as being too small and biased, and provided insufficient evidence to recommend its use.6
In summary, rapid publishing allows extensive dissemination of knowledge and sharing of experiences; yet the astute clinician needs to keep an open mind and analyse what is being published, for this cannot take the place of rigorous scientific evaluation and best clinical practice. This is a challenging time in the academic world and COVID‐19 will, no doubt, test our abilities to untangle the vast range of literature available.
- 1. Binswanger M. Excellence by nonsense: the competition for publications in modern science. In: Bartling S, Friesike S, editors. Opening science. Cham, Switzerland: Springer, 2014.
- 2. Sweileh WM. A bibliometric analysis of health‐related literature on natural disasters from 1900 to 2017. Health Res Policy Syst 2019; 17: 18.
- 3. Chiu WT, Huang JS, Ho YS. Bibliometric analysis of severe acute respiratory syndrome‐related research in the beginning stage. Scientometrics 2004; 61: 69–77.
- 4. Ioannidis JPA. Coronavirus disease 2019: the harms of exaggerated information and non‐evidence‐based measures. Eur J Clin Invest 2020; https://doi.org/10.1111/eci.13222. [Epub ahead of print]
- 5. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020; https://doi.org/10.1016/j.ijantimicag.2020.105949. [Epub ahead of print]
- 6. Taccone FS, Gorham J, Vincent JL. Hydroxychloroquine in the management of critically ill patients with COVID‐19: the need for an evidence base. Lancet Respir Med 2020 https://doi.org/10.1016/s2213-2600(20)30172-7. [Epub ahead of print]





No relevant disclosures.