Patients undergoing major orthopaedic surgery such as lower limb joint arthroplasty have traditionally been regarded as being at high risk of venous thromboembolic disease. Indeed, of patients who undergo major orthopaedic surgery without appropriate thromboprophylaxis, up to 60% will develop venous thromboembolism (VTE).1 For the most part, parenteral heparins, including the low-molecular-weight enoxaparin, have been used for thromboprophylaxis in these situations, occasionally substituted by warfarin if deemed more appropriate.2 Although heparins and warfarin both have a documented history of utility as anticoagulants in several indications, they are also associated with several clinical shortcomings. Heparins, whose use is associated with haemorrhage, require parenteral administration, which limits their application in an outpatient setting. Similarly, the use of warfarin is associated with myriad difficulties, including its delayed onset of action, its need for complex individualised dosing, its numerous interactions with food and medications, and the inherent risk of bleeding it entails.3
In light of the limitations of current anticoagulation therapies, a significant amount of research in recent years has investigated potential alternatives to warfarin and heparins. Several novel agents have undergone large-scale clinical trials to evaluate their safety and efficacy for thromboprophylaxis in the orthopaedic setting. These agents have the desirable properties of being orally active, demonstrating predictable dose–response pharmacokinetics, having a sound safety profile and yielding few drug–drug interactions. The most recent research has focused on agents that operate via the targeted inhibition of specific factors within the coagulation cascade, in particular, the inhibition of proteases such as thrombin or activated factor X (Xa). One agent that has recently emerged is rivaroxaban, an oral direct factor Xa inhibitor that has demonstrated superiority over enoxaparin in large clinical trials4 and is now approved for use in Australia. Other oral factor Xa inhibitors, such as apixaban, are currently undergoing phase III clinical trials.5
Targeted inhibition of thrombin within the coagulation cascade has been another focus of research in the investigation of novel anticoagulants. One of the early orally active thrombin inhibitors, ximelagatran, demonstrated promising safety and efficacy compared with enoxaparin for thromboprophylaxis in major orthopaedic surgery but was subsequently abandoned after it was found to cause liver dysfunction in some patients.6 More recently, clinical trials have been performed on a new oral thrombin inhibitor, dabigatran etexilate. Dabigatran etexilate has undergone large-scale international trials for orthopaedic thromboprophylaxis, demonstrating sound safety and efficacy, and is now approved for use in the United Kingdom, Europe and Canada. In November 2008, dabigatran etexilate was approved by the Therapeutic Goods Administration (TGA) for use in Australia for prevention of VTE in adults after major limb orthopaedic surgery (elective total hip or knee replacement). Here, I discuss the evidence available to support the use of dabigatran etexilate and highlight some of the potential advantages and disadvantages associated with use of this agent. Levels of evidence are provided according to the taxonomy of the National Health and Medical Research Council (NHMRC) (Box 1).7
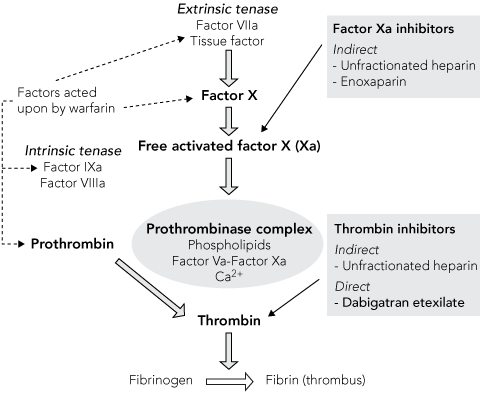
Dabigatran etexilate is a low-molecular-weight prodrug that itself exhibits no pharmacological activity. However, after oral administration, dabigatran etexilate is rapidly absorbed and converted to its active moiety, dabigatran, by catalysed hydrolysis in plasma and in the liver.8 Dabigatran is a potent, competitive and reversible direct inhibitor of the thrombin enzyme, with an oral bioavailability of 6.5%. Dabigatran has a terminal half-life of 14–17 hours, thereby facilitating once-daily dosing. The agent is eliminated primarily by renal excretion (about 80%), with the remainder conjugated and excreted via the bile. The onset of action of dabigatran is within 1 hour of dosing and the anticoagulant effects parallel plasma concentration.9 Dabigatran inhibits thrombus formation by preventing the conversion of fibrinogen into fibrin in the coagulation cascade (see Box 2). Dabigatran also inhibits free thrombin, fibrin-bound thrombin and thrombin-induced platelet aggregation.8,9 As dabigatran is orally active, has stable, predictable pharmacokinetics and can be administered without laboratory monitoring or dose titration, it affords several potential advantages in comparison with the current generation of anticoagulation therapies (Box 3).
Three large phase III clinical trials have evaluated the use of dabigatran in patients undergoing total hip or knee arthroplasty. These were powered for non-inferiority — that is, they aimed to establish whether dabigatran was no worse than enoxaparin for thromboprophylactic use in orthopaedic surgery. In two of the three trials, the primary efficacy end point was met. The primary end points for the efficacy analysis were total VTE (the composite of deep vein thrombosis [DVT], non-fatal pulmonary embolism and all-cause mortality) and the composite of major VTE (venographic or symptomatic proximal DVT and pulmonary embolism) and VTE-related mortality. The main safety end point was the frequency of major bleeding events occurring between the first dose of study medication and 3 days after the last dose. Box 4 provides a synopsis of the efficacy and safety results from these three trials.
The RE-NOVATE study randomly assigned 3494 patients undergoing total hip replacement to receive 28–35 days’ treatment with dabigatran etexilate 150 mg daily, dabigatran etexilate 220 mg daily or subcutaneous enoxaparin 40 mg daily. The dosing regimen for RE-NOVATE was such that dabigatran therapy was started with a half dose 1–4 hours after surgery — 75 mg or 110 mg for patients assigned to receive 150 mg and 220 mg, respectively — and enoxaparin therapy was started the day before surgery. For this trial, a third of the lower boundary of the 95% confidence interval, 7.7%, was chosen as a conservative estimate of the non-inferiority margin. In RE-NOVATE, both doses of dabigatran were found to be non-inferior to enoxaparin (E2).12 The primary efficacy outcome occurred in 6.7% of individuals (n = 60) in the enoxaparin group, compared with 6.0% of patients in the dabigatran 220 mg group (absolute difference 20.7%; 95% CI, 22.9% to 1.6%) and 8.6% of patients in the 150 mg group (absolute difference 1.9%; 95% CI, 20.6 to 4.4%). There was also no statistically significant difference in major bleeding rate with either dose of dabigatran compared with enoxaparin (P = 0.60 for dabigatran 150 mg; P = 0.44 for dabigatran 220 mg).
The RE-MODEL trial, a phase III study comparing two doses of dabigatran etexilate (150 mg daily and 220 mg daily) with subcutaneous enoxaparin 40 mg daily, was performed in the context of knee arthroplasty.13 In this trial, dabigatran therapy was started with a half dose 1–4 hours after surgery — 75 mg or 110 mg for patients assigned to receive 150 mg and 220 mg, respectively — and enoxaparin therapy was started the day before surgery. In this study, one-third of the lower boundary of the 95% confidence interval, 9.2%, was selected as an estimate of the non-inferiority margin. The results of RE-MODEL with respect to the primary end point (total VTE including asymptomatic VTE plus all-cause mortality) showed that dabigatran’s antithrombotic effect for both doses tested was statistically non-inferior to the effect of enoxaparin (E2). The primary efficacy outcome in RE-MODEL occurred in 40.5%, 36.4% and 37.7% of patients assigned to dabigatran etexilate 150 mg or 220 mg or enoxaparin, respectively. The rates of major bleeding were 1.3%, 1.5% and 1.3% for patients receiving dabigatran etexilate 150 mg or 220 mg or enoxaparin, respectively, and it was noted that a late, transient rise in transaminases was observed in six patients (0.5%) who had received dabigatran.13
A further study on patients undergoing knee arthroplasty, the RE-MOBILIZE trial, randomly assigned patients to receive dabigatran etexilate 150 mg daily, dabigatran etexilate 220 mg daily or subcutaneous enoxaparin 30 mg twice daily.14 An upper limit of 9.2% for the 95% confidence interval for the risk difference found between dabigatran and enoxaparin therapies for the primary efficacy outcome, was selected as the non-inferiority margin. The dosing regimen for RE-MOBILIZE involved starting dabigatran therapy at a half dose 6–12 hours after surgery — 75 mg or 110 mg for patients assigned to receive 150 mg and 220 mg, respectively — and starting enoxaparin therapy 12–24 hours after surgery. This higher dose of enoxaparin was selected as this is consistent with North American thromboprophylaxis protocols. In RE-MOBILIZE, the primary efficacy end point was not met, as dabigatran did not demonstrate non-inferiority compared with this higher dose of enoxaparin (E2). The failure of dabigatran to achieve non-inferiority with the comparator in RE-MOBILIZE was attributed to the incidence of asymptomatic distal DVT detected at the end of therapy, as major VTE occurred at a similar rate in all groups in the study.15 However, an important point to emerge from this study was that there was no difference in safety outcomes between either dose of dabigatran and enoxaparin, with a trend of less major bleeding in the dabigatran group (0.6%) than in the enoxaparin group (1.4%).16
Overall, pooled analysis of the results from these phase III studies (Box5), which involved more than 8000 patients, showed dabigatran to be comparable to enoxaparin for prevention of VTE and VTE-related mortality after both knee and hip replacement (E2).16 Pooled data analysis also revealed that dabigatran’s safety profile was comparable to enoxaparin — incidence of major bleeding, as well as secondary safety end points such as elevation of liver enzymes and treatment-emergent acute coronary syndrome events, was similar across treatment groups (E2).
Dabigatran is currently undergoing investigation for use in other clinical indications. A recent non-inferiority study, the RE-LY trial, compared two doses of dabigatran etexilate (110 mg and 150 mg, each twice daily) with therapeutic warfarin for the prevention of stroke or systemic embolism in patients with atrial fibrillation.17 This study enrolled more than 18 000 patients, and had a median follow-up period of 2 years. It showed that dabigatran etexilate administered at a dose of 110 mg was associated with rates of stroke and systemic embolism similar to therapeutic warfarin, but with lower rates of major haemorrhage than warfarin. However, when provided at a dose of 150 mg, it was associated with statistically significant lower rates of stroke and system embolism but similar rates of major haemorrhage when compared with therapeutic warfarin.
The clinical trials performed thus far have shown, for the most part, that dabigatran demonstrates a sound safety profile, is generally well tolerated and has few drug interactions. Ostensibly, as dabigatran is eliminated primarily by renal excretion, dose adjustment to 150 mg rather than a full dose of 220 mg is required in patients with impaired renal function, defined as creatinine clearance of 30–50 mL/min (E2). By extension, dabigatran is contraindicated in severe renal failure (creatinine clearance, < 30 mL/min).9
Dabigatran etexilate, the prodrug of dabigatran, is a substrate for P-glycoprotein. Accordingly, co-administration of dabigatran with strong P-glycoprotein inhibitors, such as amiodarone, quinidine, clarithromycin, verapamil and cyclosporin, should be approached with caution, and avoided if possible (E3). Additionally, close clinical surveillance is recommended for signs of bleeding or anaemia during the dabigatran treatment period. Dabigatran should not be administered concomitantly with other anticoagulants, including antithrombotics, antiplatelets and vitamin K antagonists. When dabigatran is given at recommended doses with low-dose aspirin for the prevention of cardiovascular events, there is no evidence of an excess bleeding risk9 (E3). However, clinically monitoring patients on both aspirin and dabigatran for signs of bleeding is advisable during the treatment period.
In all the phase III clinical trials of dabigatran, there was no statistically significant difference between dabigatran and enoxaparin in the incidence of abnormal liver function (E2).16 However, as dabigatran follows in the footsteps of ximelagatran, an earlier generation oral direct thrombin inhibitor that was abandoned due to the incidence of liver dysfunction in treated patients, prudent clinical practice would imply that liver function be regularly monitored for patients on dabigatran in case liver dysfunction is a class effectof thrombin inhibitors.
A summary of dabigatran’s medicinal profile is provided in Box6.
The high level of evidence in published clinical studies indicates that dabigatran is a promising anticoagulant and alternative to enoxaparin. Large-scale, international, randomised controlled trials have demonstrated that dabigatran is non-inferior to enoxaparin in the prevention of VTE after orthopaedic surgery, with a comparable safety profile (E2). In addition, as dabigatran has relatively few drug interactions (E3), it is an attractive proposition for mainstream use. Furthermore, the use of this new agent affords numerous benefits to patients and clinicians because dabigatran is orally active, does not require routine laboratory monitoring or dose adjustment, and has stable pharmacokinetics.18 Important messages for patients are shown in Box7.
Although dabigatran has demonstrated sound results in robust, well designed clinical trials, some issues need to be considered when using this agent. Importantly, dabigatran has no specific antidote, so clinicians must be vigilant in prescribing this drug, especially for use in an outpatient setting where the risk of overdose may be higher. Also, dabigatran cannot be used in patients treated with concomitant anticoagulants, which excludes a substantial proportion of patients who might otherwise benefit from the drug (E3). It is also a salient observation that, thus far, there have been no studies of dabigatran on pregnant or lactating women, patients with severe liver disease, or children. Further research and post-marketing surveillance of dabigatran is likely to determine how broadly the drug may be used. Additionally, the crucial issue of cost-effectiveness needs to be investigated. Although large studies indicate that dabigatran is cost-saving compared with enoxaparin (E1) in the context of the UK National Health Service,11 the cost-effectiveness of dabigatran in Australia remains to be determined. Additionally, dabigatran is not currently listed on the Pharmaceutical Benefits Scheme.
1 Classification of levels of evidence endorsed by the National Health and Medical Research Council (NHMRC)7
E1: evidence obtained from a systematic review of all relevant randomised controlled trials.
E2: evidence obtained from at least one properly designed randomised controlled trial.
2 Mechanism of action of dabigatran and current anticoagulants*
 |
|
* Adapted from: Brighton TA. The direct thrombin inhibitor melagatran/ximelagatran. Med J Aust 2004; 181: 432-437.10 Reproduced with permission. |
3 Comparison of dabigatran with warfarin and enoxaparin
4 Summary of phase III studies of dabigatran for prophylaxis against venous thrombosis after major orthopaedic surgery
5 Pooled analysis of venous thromboembolism (VTE) and major bleeding data from phase III studies of dabigatran16
6 Drug profile summary for dabigatran
Administration: Once daily, orally (taken with water, with or without food).
E2 = evidence obtained from at least one properly designed randomised controlled trial.7
7 Important messages for patients
Dabigatran is a drug with similar anticlotting effects to warfarin and enoxaparin.
Unlike warfarin, dabigatran does not require frequent blood tests for monitoring.
Unlike enoxaparin, dabigatran does not require administration via injection.
Dabigatran has low potential for interaction with diet and other medications.
Dabigatran may interact with some drugs, including the cardiac medications amiodarone and verapamil, the antibiotic clarithromycin, and antifungals such as ketoconazole and itraconazole.
Dabigatran is currently marketed in Australia under the name Pradaxa.
- Abhishek K Verma1,2
- 1 Gosford District Hospital, Gosford, NSW.
- 2 School of Medicine and Public Health, University of Newcastle, Newcastle, NSW.
None identified.
- 1. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 (3 Suppl): 338S-400S.
- 2. Rang HP, Dale MM, Ritter JM. Pharmacology. 4th ed. New York: Churchill Livingstone Publishers, 2000: 316-318.
- 3. Kalant H, Roschlau WHE. Principles of medical pharmacology. 6th ed. New York: Oxford University Press, 1998: 532-535.
- 4. Verma AK, Brighton TA. The direct factor Xa inhibitor rivaroxaban. Med J Aust 2009; 190: 379-383. <MJA full text>
- 5. Lassen MR, Davidson BL, Gallus A, et al. The efficacy and safety of apixaban, an oral, direct factor Xa inhibitor, as thromboprophylaxis in patients following total knee replacement. J Thromb Haemost 2007; 5: 2368-2375.
- 6. Kaul S, Diamond GA, Weintraub WS. Trials and tribulations of noninferiority: the ximelagatran experience. J Am Coll Cardiol 2005; 46: 1986-1995.
- 7. National Health and Medical Research Council. A guide to the development, evaluation and implementation of clinical practice guidelines. Canberra: NHMRC, 1999.
- 8. Stangier J, Rathgen K, Stähle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol 2007; 64: 292-303.
- 9. MIMS Australia. Abbreviated prescribing information: dabigatran etexilate (Pradaxa). Sydney: CMP Medica Australia, 2009.
- 10. Brighton TA. The direct thrombin inhibitor melagatran/ximelagatran. Med J Aust 2004; 181: 432-437. <MJA full text>
- 11. Wolowacz SE, Roskell NS, Maciver F, et al. Economic evaluation of dabigatran etexilate for the prevention of venous thromboembolism after total knee and hip replacement surgery. Clin Ther 2009; 31: 194-212.
- 12. Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet 2007; 370: 949-956.
- 13. Eriksson BI, Dahl O, Dijk V, et al. A new oral anticoagulant, dabigatran etexilate, is effective and safe in preventing venous thromboembolism after total knee replacement surgery (the RE-MODEL trial) [abstract]. Blood (ASH Annual Meeting Abstracts) 2006; 108: A573.
- 14. Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24: 1-9.
- 15. Light A. Untitled. 2007; 27 Oct. http://www.warfarinfo.com/dabigatran.htm (accessed May 2009).
- 16. Rosencher N, Bellamya L, Arnaouta L. Should new oral anticoagulants replace low-molecular-weight heparin for thromboprophylaxis in orthopaedic surgery? Arch Cardiovasc Dis 2009; 102: 327-333.
- 17. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139-1151.
- 18. Di Nisio M, Middelderp S, Buller H. Direct thrombin inhibitors. N Engl J Med 2005; 353: 1028-1040.





Abstract
Dabigatran etexilate was recently approved by the Therapeutic Goods Administration for thromboprophylactic use in adults undergoing elective total hip or knee replacement.
Dabigatran etexilate is the prodrug of the active moiety dabigatran, an orally active agent that could replace enoxaparin in some clinical indications.
Dabigatran is a direct thrombin inhibitor; it has stable, predictable pharmacokinetics and does not require routine monitoring.
Pooled efficacy data from large-scale phase III clinical trials of dabigatran use in orthopaedic thromboprophylaxis have shown non-inferiority to enoxaparin, with total venous thromboembolism results of 3.8% for dabigatran etexilate 150 mg and 3.0% for dabigatran etexilate 220 mg, compared with 3.3% for enoxaparin.
Pooled safety results for dabigatran are similar to those for enoxaparin, with major bleeding rates of 1.1% for dabigatran etexilate 150 mg and 1.4% for dabigatran etexilate 220 mg, compared with 1.4% for enoxaparin.
Dabigatran failed to demonstrate non-inferiority compared with enoxaparin 30 mg twice daily for orthopaedic thromboprophylaxis.
Issues relating to the use of dabigatran include its lack of antidote, limited application in renal disease, and interaction with drugs such as amiodarone and verapamil.
Several trials investigating the use of dabigatran for other indications, such as stroke prevention in atrial fibrillation and acute coronary syndromes, are underway.
Given its safety profile, efficacy, oral bioavailability and stable pharmacokinetic properties, dabigatran may be a viable alternative to enoxaparin for thromboprophylaxis in orthopaedic surgery.