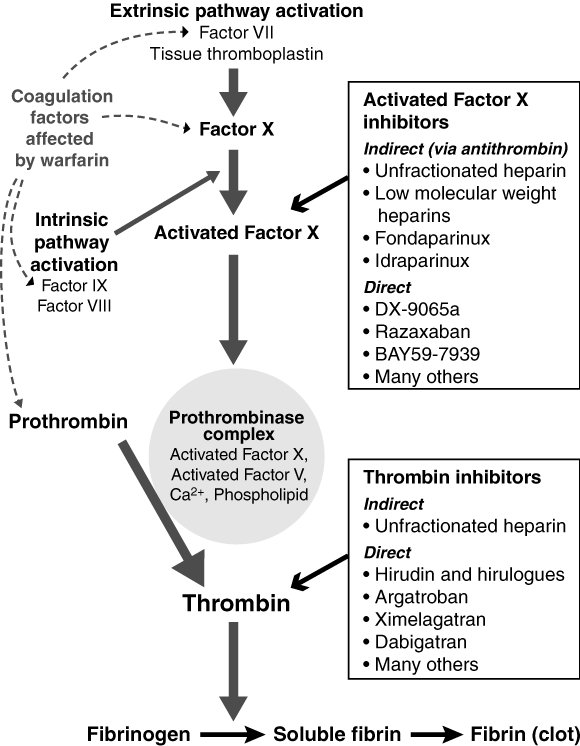
Coagulation is initiated by the tissue-factor-dependent activation of factor VII (see Box 1). Key events in the coagulation cascade are the activation of platelets, the generation of activated factor X, and the subsequent activation of prothrombin to thrombin by the prothrombinase complex. Thrombin is the key enzyme cleaving fibrinogen to produce fibrin clot. Thrombin has many other activities, including a potent positive feedback by activating factors V and VIII, further activation of platelets, stimulation of anticoagulation mechanisms, and promotion of clot dissolution and healing. A number of anticoagulant drugs targeting activated factor X or thrombin are currently marketed or in advanced stages of clinical testing (Box 1).
Ximelagatran has many desirable properties as an anticoagulant when compared with warfarin sodium (see Box 2).1 Melagatran is a potent, rapidly binding, competitive and reversible direct inhibitor of soluble and clot-bound thrombin. Oral ximelagatran has stable bioavailability and is rapidly converted to melagatran, with peak plasma melagatran concentrations 1.5–2.5 hours after oral dosing. Absorption is minimally influenced by food and other medications. Melagatran has no significant protein binding, is not significantly metabolised, and 80% is excreted renally over 24 hours. The half-life of melagatran is relatively short at 1.5–2 hours in young, healthy people, necessitating twice-daily dosing. Because of the age-related decrease in renal function, the plasma half-life of melagatran increases with patient age; its half-life in a typical 70-year-old patient is 4–5 hours. In patients with severe renal impairment, the plasma half-life of melagatran is significantly longer.2 The impact of renal impairment on dosing and adverse effects needs to be explored in greater detail, although there was no significant accumulation with repeated dosing in healthy young or elderly individuals. Doses of melagatran/ximelagatran have varied in different clinical indications. Maximal plasma concentration of 600 nmol/L is achieved after 5 mg subcutaneous melagatran or 60 mg of oral ximelagatran. Ximelagatran administration at therapeutic doses results in significant prolongations of clotting times (eg, activated partial thromboplastin time, prothrombin time and thrombin time).
In clinical studies, over 17 000 patients have received melagatran/ximelagatran and no observed interactions with concomitant medications have been noted. Preliminary studies of possible interactions of melagatran/ximelagatran with alcohol or specific medications (including nifedipine, diazepam, diclofenac, acetylsalicylic acid, digoxin and statins) have found none, as melagatran/ximelagatran metabolism is independent of CYP450 enzymes (abstract presentations only). One study found that concomitant ximelagatran and erythromycin increased maximal plasma concentration of melagatran in healthy volunteers by almost twofold.
Excluding haemorrhage (discussed below), melagatran/ximelagatran has been remarkably free of significant adverse effects. Most attention has focused on the dose-dependent increase in liver enzyme levels (predominantly alanine aminotransferase [ALT]), which is usually asymptomatic and occurs in 6%–12% of patients. This biochemical abnormality typically develops between the first and sixth month of therapy, but rarely after 6 months. The proportion of patients with ximelagatran-induced increased liver enzyme concentrations who become jaundiced or develop symptoms attributable to liver dysfunction appears to be small (< 1%–2%), but precise details have not yet been published. All patients taking melagatran/ximelagtran require monthly liver function testing for the first 6 months. Therapy should be ceased if the ALT concentration exceeds five times the upper limit of normal at any time. More frequent monitoring is required in patients in whom ALT concentrations increase to 3–5 times the upper limit of normal. The biochemical abnormality usually (> 95% of occasions) completely resolves with either continuation or discontinuation of the medication. The mechanism of the liver enzyme abnormality remains unclear. The importance of concurrent liver disease and concomitant use of other liver toxins remains to be determined.
The clinical efficacy and safety of melagatran/ximelagatran has been assessed in an expansive clinical study program. These have focused on stroke prevention in non-valvular atrial fibrillation, prophylaxis of vein thrombosis after orthopaedic surgery, acute therapy of vein thrombosis, secondary prevention of recurrent vein thrombosis, and acute coronary syndromes. In this article ximelagatran refers to the combination of melagatran and ximelagatran unless otherwise specified.
Non-valvular atrial fibrillation (NVAF) affects 6% of people over the age of 65 years and about 10% of those over 80. Patients with NVAF have an absolute risk of stroke and systemic embolism of around 5%, which varies with age and other risk factors. Clinical studies have conclusively shown that warfarin, given to achieve a target INR of 2–3, is highly effective at preventing stroke (risk reduction of 62% in all risk groups), but at a cost of around 1% per year of fatal haemorrhage. Despite the published efficacy of warfarin, studies consistently show that only 20%–40% of eligible patients actually receive warfarin.4
Ximelagatran has been evaluated in two dose-finding exploratory studies (SPORTIF II5 and SPORTIF IV [unpublished]) and two phase III clinical studies (SPORTIF III6 and SPORTIF V7). The SPORTIF III and V studies recruited over 5000 patients with NVAF at moderate risk of stroke or systemic embolism (see Box 3). Their objective was to establish that fixed-dose ximelagatran (36 mg twice daily) was not inferior to warfarin (target INR, 2–3).8 Absolute rates of stroke and systemic embolism for patients treated with warfarin and ximelagatran were equivalent. Rates of major or fatal bleeding were not significantly different, although there was a small but significant excess of total bleeding in patients treated with warfarin. Abnormal ALT concentrations (above three times upper limit of normal) occurred in 1% of patients treated with warfarin and 6% of those treated with ximelagatran. These studies concluded that ximelagatran (36 mg twice daily) is not inferior to warfarin (target INR, 2–3) for preventing stroke, with no difference in significant bleeding rates (E2). (Levels of evidence are explained in a National Health and Medical Research Council publication.24)
Without prophylaxis, arthroplasty of the knee and hip carries unacceptable risks of postoperative vein thrombosis and death from pulmonary embolism.9 Standard prophylaxis includes early mobilisation, compression stockings and once-daily injections of low-molecular-weight heparin (LMWH) for 5–10 days. A proportion of patients undergoing knee-replacement surgery receive prophylaxis with warfarin rather than LMWH. With this prophylaxis 1%–2% of patients will still develop symptomatic deep vein thrombosis (DVT) or pulmonary embolism (PE), with fatality rates of 0.1%–0.5% over the 6-week postoperative period. Most of these clinical events occur out of hospital. Recent studies of prolonged prophylaxis with LMWH for 4–6 weeks postoperatively significantly reduce the incidence of symptomatic venous thromboembolism.10
The high rates of venous thromboembolism after major orthopaedic surgery provide an opportunity for more effective anticoagulants to improve clinical outcomes. However, clinical studies based on symptomatic events alone are difficult to perform, as the number of participants needed to achieve adequate statistical power is immense. Most studies use bilateral contrast venography performed at 7–14 days after surgery as a validated surrogate outcome. With standard prophylaxis, such venography typically finds total DVT rates of 15% after hip replacement and 30% after knee replacement, and proximal DVT rates of about 6% in both groups. Recent studies incorporating venographic endpoints have shown impressive superiority of fondaparinux over standard LMWH prophylaxis.11
Ximelagatran has been extensively studied after joint-replacement surgery. The dose-ranging pilot study (METHRO I12) and phase II study (METHRO II13) found an overall dose-dependent decrease in total and proximal DVT on venography and/or symptomatic pulmonary embolism with increasing doses of ximelagatran. The phase III studies of ximelagatran are summarised in Box 4. No difference in the rates of proximal asymptomatic DVT on venography, symptomatic vein thrombosis or major bleeding were shown between patients treated with ximelagatran and enoxaparin.14-17 Total rates of DVT on venography were significantly reduced in patients treated with ximelagatran compared with those treated with enoxaparin in the EXPRESS study15 and those treated with warfarin in the EXULT studies.16,17 These studies concluded that ximelagatran is an effective agent for preventing venous thromboembolism after major orthopaedic surgery (E2). Ximelagatran has recently been approved in 13 European countries for orthopaedic prophylaxis.
Acute treatment of acute venous thromboembolism, either DVT or PE, requires initial anticoagulation with LMWH and subsequent warfarin therapy in most patients. Most patients can be treated safely out of hospital. Warfarin therapy is usually initiated within 24–48 hours, but therapeutic anticoagulation (as measured by INR) is not achieved for about 1 week. Heparin and warfarin should be given concurrently for at least 5 days, and heparin treatment should not be ceased until INR measurements have been in the target range of 2–3 for 2 consecutive days.
The optimal duration of warfarin therapy is uncertain. In most patients with their first provoked (eg, postoperative) DVT or PE, 3–6 months is adequate. Patients with a first unprovoked proximal DVT or PE usually receive 6 months of warfarin therapy. Patients with unprovoked DVT or PE have a high rate of recurrent vein thrombosis (about 1 in 3 over 10 years). A number of studies have confirmed that extending warfarin therapy (target INR, 2–3) beyond 3–6 months is highly (≥ 95%) effective for preventing recurrent thrombosis. Unfortunately, the unpredictable risk of major or fatal haemorrhage all but eliminates the benefit for extended warfarin therapy. Extended warfarin therapy is generally reserved for patients with first unprovoked DVT/PE and significant thrombophilia (eg, malignant disease) or recurrent vein thrombosis.
Ximelagatran has been compared with standard therapy for acute treatment of DVT with or without asymptomatic PE (THRIVE I,18 THRIVE II [unpublished], THRIVE IV19 and THRIVE V20), and with placebo for extended secondary prophylaxis (THRIVE III21). The details of these studies are summarised in Box 5. The combined results of the THRIVE II and V studies found that ximelagatran (36 mg twice daily for 6 months) was not inferior to standard therapy (enoxaparin and warfarin [targeted INR, 2–3] for 6 months) (E2).20 There was no difference in major haemorrhage or all-cause mortality between patients treated with ximelagatran or warfarin.
In the THRIVE III study of extended prophylaxis, 1233 patients were randomly assigned in a double-blind fashion to receive placebo or ximelagatran (24 mg twice daily) for 18 months after completing 6 months of anticoagulation therapy for acute DVT or PE.21 The cumulative risk of symptomatic recurrent vein thrombosis was 2.8% in patients treated with ximelagatran and 12.6% in those taking placebo (risk reduction, 0.84; 95% CI, 0.7–0.91; P < 0.001). Major haemorrhage occurred in 6 of 612 patients treated with ximelagatran and 7 of 611 patients given placebo. While ximelagatran is clearly superior to placebo for secondary prophylaxis (E2), identical results are obtained when placebo and warfarin therapy are compared. The haemorrhagic potential of ximelagatran compared with warfarin in this setting remains to be clarified.
Recent studies have shown that warfarin, or warfarin combined with low-dose aspirin, is effective in patients with acute coronary disease for preventing recurrent ischaemia, reinfarction and death.22 A single phase II study, the ESTEEM study, compared (in patients all taking 160 mg aspirin once daily) placebo with varying doses of ximelagatran (24, 36, 48 or 60 mg twice daily).23 Overall, there was a 20% reduction in a composite endpoint of recurrent non-fatal myocardial infarction, recurrent ischaemia or death from any cause in patients treated with ximelagatran. Major bleeding occurred in 1.8% of the combined ximelagatran group and 0.9% of patients treated with aspirin only (difference not statistically significant). These results are equivalent to those seen with warfarin or warfarin combined with aspirin,21 but more studies are required before firm conclusions can be reached (E3).
The clinical studies of ximelagatran confirm that it is an effective antithrombotic agent in stroke prevention in non-valvular atrial fibrillation (E2), prevention of vein thrombosis after major orthopaedic surgery (E2), acute therapy of DVT (E2) and extended prevention of recurrent vein thrombosis (E2), and possibly in preventing recurrent ischaemia after acute myocardial infarction (E3). In most indications, ximelagatran was as effective as standard therapy and had equivalent propensity to cause bleeding. In major arthroplasty ximelagatran was more effective than standard therapy as prophylaxis against vein thrombosis. Important information for patients is shown in Box 6.
Patients taking ximelagatran will require monthly blood tests for liver biochemistry for 6 months. Some 6%–12% of patients treated with ximelagatran will develop asymptomatic, but significantly abnormal, ALT levels, leading to discontinuation of ximelagatran therapy in about half of cases. While serious or symptomatic liver toxicity occurred very infrequently in the clinical studies, questions remain about the mechanism of toxicity and implications for wider community use.
A number of important patient groups have been excluded from the clinical evaluation of ximelagatran (pregnant and lactating women, and patients with a serum creatinine level > 150 μmol/L, abnormal liver enzyme levels, or symptomatic pulmonary embolism). Further study in patients with varying degrees of renal or liver dysfunction is required.
A number of new anticoagulant medications will emerge over the next 5–10 years for patients with thrombotic disease. For example, in orthopaedic surgery, new agents or an extension of standard prophylaxis with LMWH for 4 weeks has already been established as significantly superior to standard 7–10-day postoperative prophylaxis. Nevertheless, ximelagatran is an exciting new oral antithrombotic drug which it is hoped will benefit many patients with venous and arterial thromboembolic disease.
2 Comparison of melagatran/ximelagatran and warfarin sodium
Property |
Warfarin sodium |
Melagatran/ximelagatran |
|||||||||||||
Mechanism of action |
Reduced synthesis of functional prothrombin and other clotting factors |
Direct competitive and reversible inhibition of soluble and clot-bound thrombin |
|||||||||||||
Rapid onset of action |
No |
Yes |
|||||||||||||
Effective anticoagulant |
Yes |
Yes (not inferior to well-controlled warfarin therapy in most studies) |
|||||||||||||
Risk of haemorrhage |
Significant |
Equivalent to warfarin in most studies |
|||||||||||||
Route of administration |
Oral, once daily |
Oral, twice daily |
|||||||||||||
Stable, predictable pharmacokinetics |
No |
Yes |
|||||||||||||
Interactions with diet and alcohol |
Clinically significant |
No |
|||||||||||||
Interactions with other medications |
Many |
Low potential |
|||||||||||||
Dosing |
Individualised to each patient and a target INR |
Fixed dosing dependent on indication |
|||||||||||||
Monitoring |
International normalised ratio (INR) every 1–2 weeks |
No monitoring of anticoagulant level. Monthly liver enzyme tests for first 6 months |
|||||||||||||
Dose adjustments |
Frequent |
No |
|||||||||||||
Use in severe liver disease |
Problematic |
Unknown (such patients excluded from studies) |
|||||||||||||
Use in severe renal disease |
Yes |
No. Melagatran is excreted renally, and patients with renal disease were excluded from clinical studies |
|||||||||||||
Reversibility after cessation |
Slow elimination and reversal of antithrombotic effect |
Reversal of thrombin inhibition dependent on plasma concentration and elimination half-life (approx. 4 h) |
|||||||||||||
Antidote |
Rapid reversal with plasma or factor replacement |
None available. Plasma or factor replacement not effective. Possible removal by dialysis |
|||||||||||||
Drug cost |
Cheap |
Unknown |
|||||||||||||
3 Summary of phase III studies of ximelagatran versus warfarin for stroke prevention in non-valvular atrial fibrillation*
Study |
SPORTIF III |
SPORTIF V |
|||||||||||||
Design |
RCT open-label |
RCT double-blind |
|||||||||||||
Study interventions |
|||||||||||||||
Ximelagatran |
36 mg twice daily |
36 mg twice daily |
|||||||||||||
Warfarin |
Target INR, 2–3 |
Target INR, 2–3 |
|||||||||||||
Number of patients |
|||||||||||||||
Ximelagatran |
1472 |
1960 |
|||||||||||||
Warfarin |
1452 |
1962 |
|||||||||||||
Absolute risk of stroke + systemic embolism† |
|||||||||||||||
Ximelagatran |
1.6% |
1.6% |
|||||||||||||
Warfarin |
2.3% |
1.2% |
|||||||||||||
Absolute risk of fatal or major bleeding† |
|||||||||||||||
Ximelagatran |
1.3% |
2.4% |
|||||||||||||
Warfarin |
1.8% |
3.1% |
|||||||||||||
% of patients with ALT level > 3 times upper limit of normal‡ |
|||||||||||||||
Ximelagatran |
6% |
6% |
|||||||||||||
Warfarin |
0.8% |
1.0% |
|||||||||||||
In SPORTIF III, medication discontinuation rates were 3.2% for patients taking ximelagatran, and 0.3% for those taking warfarin. Discontinuation rates were not published for SPORTIF IV. INR = international normalised ratio. ALT = alanine aminotransferase. * Patients were at moderate risk of stroke or systemic embolism as defined by at least one additional risk factor for stroke, including previous stroke, previous systemic embolism, age > 65 years, hypertension, diabetes, coronary artery disease, or left ventricular dysfunction (as defined by a left ventricular ejection fraction < 40%), or symptomatic congestive heart failure within 3 months. † Difference between ximelagatran and warfarin not significant. ‡ Difference between ximelagatran and control therapy significant at P < 0.05. |
|||||||||||||||
4 Summary of phase III studies of ximelagatran for prophylaxis against vein thrombosis after major orthopaedic surgery
Study |
METHRO III |
EXPRESS |
EXULT A |
EXULT B |
|||||||||||
Design |
Randomised double blind |
Randomised double blind |
|||||||||||||
Type of surgery |
Hip and knee replacement |
Knee replacement |
|||||||||||||
Study interventions |
|||||||||||||||
Control |
Enoxaparin (40 mg) 12 h before surgery; then once daily after surgery for 8–11 days |
Warfarin initiated evening after surgery and adjusted to INR 2.5 |
|||||||||||||
Ximelagatran |
Melagatran 3 mg 4–8 h after surgery; then ximelagatran 24 mg twice daily for 8–11 days |
Melagatran 2 mg at knife-to-skin; melagatran 3 mg 8 h after surgery, then ximelagatran 24 mg twice daily for 8–11 days |
Ximelagatran 24 mg or 36 mg initiated 12 h after surgery; then twice daily for 7–12 days |
Ximelagatran 36 mg twice daily initiated morning after surgery for 7–12 days |
|||||||||||
Numbers of patients |
|||||||||||||||
Ximelagatran (control) |
1399 (1389)* |
1410 (1425)* |
614 (24 mg) 629 (36 mg) (608)* |
1151 (1148)* |
|||||||||||
Total venous thromboembolism |
|||||||||||||||
Ximelagatran (control) |
31.0% (27.3%)* |
20.3% (26.6%)† |
24.9% (24 mg) 20.3% (36 mg) (27.6%)† |
22.5% (31.9%)† |
|||||||||||
Major venous thromboembolism |
|||||||||||||||
Ximelagatran (control) |
5.7% (6.2%)* |
2.3% (6.3%)† |
2.5% (24 mg) 2.7% (36 mg) (4.1%)* |
3.9% (4.1%)* |
|||||||||||
Fatal or major bleeding |
|||||||||||||||
Ximelagatran (control) |
1.6% (1.7%)* |
3.3% (1.2%)* |
0.8% (0.7%)* |
1.0% (0.4%)* |
|||||||||||
INR = international normalised ratio. Total venous thromboembolism = Distal plus proximal deep vein thrombosis on venography plus fatal or non-fatal pulmonary embolism plus death from any cause during treatment period. Major venous thromboembolism = Proximal deep vein thrombosis on venography plus fatal or non-fatal pulmonary embolism plus unexplained death during treatment period where pulmonary embolism could not be excluded. * Difference between ximelagatran and control therapy not significant. † Difference between ximelagatran and control therapy significant at P < 0.05. |
|||||||||||||||
5 Summary of phase III studies of ximelagatran for treatment of deep vein thrombosis
Study |
THRIVE II and IV |
THRIVE III |
|||||||||||||
Design |
Randomised double-blind |
Randomised double-blind |
|||||||||||||
Indication |
Acute therapy for proximal DVT |
Extended secondary prevention of DVT |
|||||||||||||
Interventions |
|
|
|||||||||||||
Control |
Enoxaparin + warfarin (INR, 2–3) for 6 months |
Placebo for 18 months |
|||||||||||||
Ximelagatran |
36 mg twice daily for 6 months |
24 mg twice daily for 18 months |
|||||||||||||
Number of patients |
|||||||||||||||
Ximelagatran (control) |
1240 (1249)* |
612 (611)* |
|||||||||||||
Recurrent symptomatic vein thrombosis |
|||||||||||||||
Ximelagatran (control) |
2.1% (2.0%)* |
2.8% (12.6%)† |
|||||||||||||
Deaths |
|
|
|||||||||||||
Ximelagatran (control) |
2.3% (3.4%)* |
1.1% (1.4%)* |
|||||||||||||
Fatal or major bleeding |
|||||||||||||||
Ximelagatran (control) |
1.3% (2.2%)* |
1.1% (1.3%)* |
|||||||||||||
% of patients with ALT level > 3 times upper limit of normal |
|||||||||||||||
Ximelagatran (control) |
9.6% (2.0%)* |
6.0% (1.0%)* |
|||||||||||||
In THRIVE III, 2.1% of patients taking ximelagatran discontinued their medication. Discontinuation rates were not published for the placebo in THRIVE III, or for ximelagatran or the control in THRIVE II and IV.
|
|||||||||||||||
6 Important messages for patients
Ximelagatran is a new oral anticoagulant drug with similar effects to those of warfarin.
Use of ximelagatran does not require frequent blood testing to check the dose, but monthly blood tests will be required to monitor liver function.
Ximelagatran has low potential for interactions with diet and other medications.
Ximelagtran is not yet marketed in Australia.
- 1. Gustafsson D, Elg M. The pharmacodynamics and pharmacokinetics of the oral direct thrombin inhibitor ximelagatran and its active metabolite melagatran: a mini-review. Thrombosis Res 2003; 109: S9-S16.
- 2. Eriksson UG, Johansson S, Attman P, et al. Influence of severe renal impairment on the pharmacokinetics and pharmacodynamics of oral ximelagatran and subcutaneous melagatran. Clin Pharmacokinet 2003; 42: 743-753.
- 3. Dorani H, Schützer K, Sarich T, et al. The pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor ximelagatran when administered with erythromycin. Poster PO08, presented at the 18th International Congress on Thrombosis. Available at: www.thrombosis2004.org/sub/cont-po.htm (accessed Aug 2004).
- 4. Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med 2000; 160: 41-46.
- 5. Petersen P, Grind M, Adler J. Ximelagatran versus warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. SPORTIF II: a dose-guiding, tolerability, and safety study. J Am Coll Cardiol 2003; 41: 1445-1451.
- 6. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non-valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet 2003; 362: 1691-1698.
- 7. Stroke prevention using the oral direct thrombin inhibitor ximelagatran in patients with nonvalvular atrial fibrillation (SPORTIF V). Circulation 2003; 108: 2723.
- 8. Halperin JL. Ximelagatran compared with warfarin for prevention of thromboembolism in patients with nonvalvular atrial fibrillation: Rationale, objectives, and design of a pair of clinical studies and baseline patient characteristics (SPORTIF III and V). Am Heart J 2003; 146 : 431-438.
- 9. Geerts WH, Heit JA, Clagett GP, et al Prevention of venous thromboembolism. Chest 2001; 119: 132S-175S.
- 10. Eikelboom JW, Quinlan DJ, Douketis JD. Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomised trials. Lancet 2001; 358: 9-15.
- 11. Bounameaux H, Perneger T. Fonfaparinux: a new synthetic pentasaccharide for thrombosis prevention [Commentary]. Lancet 2002; 359: 1710-1711.
- 12. Eriksson BI, Arfwidsson A-C, Frison L, et al. A dose-ranging study of the oral direct thrombin inhibitor, ximelagatran, and its subcutaneous form, melagatran, compared with dalteparin in the prophylaxis of thromboembolism after hip or knee replacement. METHRO I. Thromb Haemost 2002; 87: 231-237.
- 13. Eriksson BI, Bergqvist D, Kalebo P, et al. Ximelagatran and melagatran compared with dalteparin for prevention of venous thromboembolism after total hip or knee replacement: the METHRO II randomised trial. Lancet 2002; 360: 1441-1447.
- 14. Eriksson BI, Agnelli G, Cohen AT, et al; METHRO III Study Group. Direct thrombin inhibitor melagatran followed by oral ximelagatran in comparison with enoxaparin for prevention of venous thromboembolism after total hip or knee replacement. Thromb Haemost 2003; 89: 288-296.
- 15. Eriksson BI, Agnelli G, Cohen AT, et al. The direct thrombin inhibitor melagatran followed by oral ximelagatran compared with enoxaparin for the prevention of venous thromboembolism after total hip or knee replacement: the EXPRESS study. J Thromb Haemost 2003; 1: 2490-2496.
- 16. Francis CW, Berkowitz SD, Comp PC, et al. Comparison of ximelagatran with warfarin for the prevention of venous thromboembolism after total knee replacement (Exult A study). N Engl J Med 2003; 349: 1703-1712.
- 17. Colwell CW, Berkowitz SD, Comp PC, et al. Randomised double-blind comparison of ximelagatran, an oral direct thrombin inhibitor, and warfarin to prevent venous thromboembolism after total knee replacement (Exult B study). Blood 2003; 102: 14a.
- 18. Eriksson H, Wahlander K, Gustafsson D, et al. A randomised, controlled, dose-guiding study of the oral direct thrombin inhibitor ximelagatran compared with standard therapy for the treatment of acute deep vein thrombosis: THRIVE I. J Thromb Haemost 2003; 1: 41-47.
- 19. Walhander K, Lapidus L, Olsson CG, et al. Pharmacokinetics, pharmacodynamics and clinical effects of the oral direct thrombin inhibitor ximelagatran in acute treatment of patients with pulmonary embolism and deep vein thrombosis. Thromb Res 2002; 107: 93-99.
- 20. Francis CW, Ginsberg JS, Berkowitz SD, et al. Efficacy and safety of the oral direct thrombin inhibitor ximelagatran compared with current standard therapy for acute, symptomatic deep vein thrombosis, with or without pulmonary embolism: The THRIVE Treatment Study. Blood 2003; 102: 6a.
- 21. Schulman S, Wahlander K, Lundstrom T, et al. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran (THRIVE III). N Engl J Med 2003; 349: 1713-1721.
- 22. Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002; 347: 969-974.
- 23. Wallentin L, Wilcox RG, Weaver WD, et al. Oral ximelagatran for secondary prophylaxis after myocardial infarction: the ESTEEM randomised controlled trial. Lancet 2003; 362: 789-797.
- 24. National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: NHMRC, AusInfo, 1999.





Abstract
Melagatran is a synthetic, small-peptide direct thrombin inhibitor with anticoagulant activity.
Ximelagatran, an oral prodrug, undergoes rapid enzymatic conversion to melagatran.
Melagatran has rapid onset of action, fixed twice-daily dosing, stable absorption, apparent low potential for medication interactions, and no requirement for monitoring drug levels or dose adjustment. There is no specific antidote, but the drug has a short plasma elimination half-life (about 4 hours).
In clinical studies, melagatran/ximelagatran is not inferior to warfarin for stroke prevention in patients with non-valvular atrial fibrillation, to heparin–warfarin for acute treatment and extended secondary prevention of deep vein thrombosis, and superior to warfarin for prevention of venous thromboembolism after major orthopaedic surgery. Major bleeding with melagatran/ximelagatran occurred at rates similar to those in patients treated with warfarin.
6%–12% of patients taking ximelagatran develop asymptomatic elevated liver enzyme levels (predominantly alanine aminotransferase) after 1–6 months of therapy; this usually resolves with cessation of therapy. Less than 1% of patients develop abnormal liver function while taking ximelagatran; this rarely persists or develops into clinical illness.