Since the 1920s, when heparin was discovered by a medical student in liver cell extracts and warfarin was synthesised from the dicoumarol found in spoiled cattle feed, these two agents have remained the mainstays of anticoagulation treatment. The broad range of indications for these medications, including prophylaxis of thromboembolic disease in surgical patients, as well as in patients with atrial fibrillation, prosthetic heart valves or unstable coronary syndromes,1 has made these drugs frequently prescribed among patients aged 65 years or older. Unfractionated heparin has now been superseded by its low-molecular-weight counterparts (such as enoxaparin) in many clinical applications, and, although they facilitate outpatient therapy, there are significant limitations and complications of both heparin and warfarin therapies.2 Heparins require parenteral administration and their use is associated with haemorrhage. Similarly, the risks of bleeding, the delayed onset of action, the numerous interactions with food and other medications, and the complexity of individualised dosing make warfarin a dangerous and inconvenient medication for patients and their treating doctors.3,4
One novel anticoagulant to emerge recently was ximelagatran, an orally active direct thrombin inhibitor. Clinical trials demonstrated that ximelagatran’s effectiveness in preventing strokes in patients with atrial fibrillation was similar to warfarin, with suggestions that it offered a comparable safety profile, as well as easier dose administration and a decreased need for monitoring.5 However, ximelagatran was dramatically withdrawn from the market in February 2006 when it was shown that some 6% of patients demonstrated abnormal liver functions subsequent to therapy.6 Other drugs that also inhibit thrombin have emerged, such as dabigatran etexilate, and this drug is currently in advanced clinical trials and limited clinical use in some countries.7-9
Rivaroxaban is an oxazolidinone derivative that is a potent and selective direct inhibitor of factor Xa (see Box 1), binding the enzyme competitively and reversibly. Inhibition of factor Xa increases clotting times and decreases the formation of thrombin, the pivotal enzyme required for generation of fibrin, platelet activation and thrombus formation in venous thromboembolic disease and deep vein thrombosis (DVT).11 Rivaroxaban has an oral bioavailability of about 80% and is well absorbed from the gastrointestinal tract, with food having no major effect on its absorption.12 Plasma levels of rivaroxaban peak around 2.5–4 hours, and the drug has a terminal half-life of about 5–9 hours in young people and 11–13 hours in older people.13 Rivaroxaban exhibits dual excretion, primarily by the kidneys (66%) but also by the biliary-faecal route (28%).12,13
The espoused advantages of rivaroxaban, although similar to many novel anticoagulants in development, are numerous compared with warfarin and heparins (Box 2). Rivaroxaban has a rapid onset of action, high oral bioavailability (thus avoiding parenteral administration), and has predictable pharmacokinetic properties. Rivaroxaban can be administered without concurrent heparin, once daily in a fixed dose, without the need for individual monitoring and dose titration.3,11-13
The phase III RECORD (Regulation of Coagulation in Orthopaedic Surgery to Prevent DVT and Pulmonary Embolism [PE]) trials compared rivaroxaban with the standard treatment for venous thromboembolism (VTE), enoxaparin.14-16 The RECORD trials were randomised, double-blinded, double-dummy studies that evaluated the safety and efficacy of rivaroxaban in the prevention of VTE in patients undergoing major orthopaedic surgery (hip or total knee arthroplasty) (Box 3). The primary efficacy endpoint in each trial was a composite of DVT (either symptomatic or detected by bilateral venography), non-fatal PE, or death from any cause at follow-up (range, 13–42 days). The primary safety endpoint was the incidence of major bleeding.
All three RECORD trials found a lower incidence of VTE in the rivaroxaban group compared with the enoxaparin group, and statistically significant reductions in absolute risk of VTE with rivaroxaban (Box 3).14-16 The RECORD 1 and 3 trials found that rivaroxaban reduced the relative risk of VTE compared with enoxaparin.14,16 The RECORD 2 trial aimed to compare extended thromboprophylaxis with rivaroxaban (oral dose of 10 mg rivaroxaban once daily for 31–39 days, with placebo injection for 10–14 days) against short-term thromboprophylaxis with enoxaparin (40 mg enoxaparin subcutaneously once daily for 10–14 days, with placebo tablet for 31–39 days) in patients who had undergone total hip arthroplasty. The results (Box 3) allowed investigators to conclude that extended thromboprophylaxis with rivaroxaban was significantly more effective than short-term enoxaparin plus placebo for the prevention of VTE, including symptomatic events, in patients undergoing total hip arthroplasty (E2).15
A pair of phase II trials, the ODIXa-DVT study and the EINSTEIN-DVT trial, investigated twice-daily and once-daily dosing of rivaroxaban, respectively, in the treatment of symptomatic proximal DVT.17-19 Each of these studies was a prospective, randomised, double-blinded, multicentre trial that compared the efficacy and safety of rivaroxaban against standard therapy for VTE — low-molecular-weight heparin and a vitamin K antagonist (VKA). Each study enrolled more than 500 patients.
The ODIXa-DVT study compared various doses of oral rivaroxaban (2.5, 5, 10, 20 and 30 mg, twice daily) with 40 mg subcutaneous enoxaparin followed by a VKA.17,18 The primary efficacy endpoint was the reduction in thrombotic burden, assessed at Day 21 by compression ultrasonography. The primary safety endpoint was the incidence of major bleeding. The results of the ODIXa-DVT trial showed that the primary efficacy endpoints for the tested doses of rivaroxaban ranged between 7% and 18%, compared with 17% for the combined enoxaparin–VKA group. In terms of the safety endpoint, it was shown that as the dose of rivaroxaban increased, so did the frequency of major bleeding complications (P = 0.045). However, the study found that there was no statistically significant difference in the rate of bleeding complications between rivaroxaban (occurring in 2.9%–7.5% of patients) and enoxaparin–VKA (occurring in 8.8% of patients) (E2).17
The EINSTEIN-DVT trial, which was a partner phase II study and similar in design to the ODIXa-DVT trial, evaluated once-daily dosing of rivaroxaban against 40 mg enoxaparin and a VKA for treatment of VTE.19 The efficacy endpoint was the deterioration in thrombotic burden, which was assessed by an ultrasound and perfusion lung scan performed at 12 weeks and compared with the results obtained at baseline. The EINSTEIN-DVT trial demonstrated this efficacy outcome in 5.4%–6.6% of patients randomly assigned to receive rivaroxaban, compared with 9.9% of those given enoxaparin–VKA. This trial again demonstrated that rivaroxaban has highly comparable efficacy to the current standard treatment for VTE. However, investigators also made the important observation that there appeared to be a decreased incidence of bleeding associated with once-daily dosing, and the optimal dose for safety and efficacy was between 15 mg and 20 mg administered once daily (E2).17,19 As a result of this performance in phase II trials, a 20 mg once-daily dose of rivaroxaban is currently being evaluated in phase III trials for the treatment of patients with symptomatic DVT or PE.
While the efficacy and safety of rivaroxaban have been demonstrated by phase III trials, there have also been numerous studies to assess potential interactions of rivaroxaban with foods and other drugs. Kubitza and colleagues performed four randomised studies, two of which assessed the interaction of rivaroxaban with food.20 No significant food–drug interactions were found, nor were there any major disturbances to any of the pharmacological parameters studied (E3).20
In another phase II study performed by the same researchers, the safety, tolerability and pharmacokinetics of the combination of rivaroxaban and the non-steroidal anti-inflammatory drug (NSAID) naproxen were investigated.21 Patients were allocated to receive naproxen alone, rivaroxaban alone or a combination of both drugs. The results demonstrated no interactions in the mechanisms between rivaroxaban and naproxen, and also showed that the addition of naproxen did not affect prothrombin time or platelet aggregation, although a significant increase in bleeding was observed in the combination group. However, this increase in bleeding was less than when naproxen was administered alone, and further studies are underway to confirm the safety of this drug combination.21
An important phase I trial was performed to determine if aspirin influenced the safety and efficacy of rivaroxaban.22 In all the subjects studied, this combination was tolerated, and aspirin did not affect either the factor Xa inhibition activity of rivaroxaban or prolongation of the prothrombin time. Additionally, it was determined that rivaroxaban did not interfere with the effects of aspirin on platelet aggregation, suggesting this combination should pose no appreciable clinical interaction (E3).22
Clinical trials undertaken thus far to assess potential interactions of rivaroxaban with common drugs have found no significant interactions with aspirin, NSAIDs, antacids, histamine H2-receptor antagonists or digoxin (E3).20-22 However, recent evidence has shown that rivaroxaban demonstrates pharmacological interactions with potent inhibitors of cytochrome P450 3A4, including drugs such as ketaconazole, macrolides (such as clarithromycin) and protease inhibitors (such as the antiretroviral drug atazanavir).23 Further studies are required to determine potential interactions between rivaroxaban and other drugs, but the results so far demonstrate that rivaroxaban appears to be largely clinically safe (E3).
Given that research to date has shown rivaroxaban to be a viable anticoagulant, the manufacturers have sought licensing approval in Europe. As licensing in Australia may soon follow, it is important that all clinicians treating patients with anticoagulants be aware of the potential advantages of rivaroxaban. A summary of rivaroxaban’s medicinal profile is provided in Box 4.
The ease of dosing and administration and decreased need for monitoring of patients treated with rivaroxaban, combined with superior outcomes, make it an attractive anticoagulant for the future. Clinical trials are underway studying the use of rivaroxaban for various indications, such as the treatment of vein thromboses and the prevention of stroke in patients with atrial fibrillation and acute coronary syndromes, and it is hoped that rivaroxaban will reduce the burden of disease caused by these conditions. However, in the interim, rivaroxaban has been proven to be a highly effective and exciting new oral anticoagulant drug. With the concurrent development of other orally active direct factor Xa inhibitors, such as apixaban, rivaroxaban will potentially benefit many patients at risk of venous thromboembolic disease. Important messages for patients are shown in Box 5.
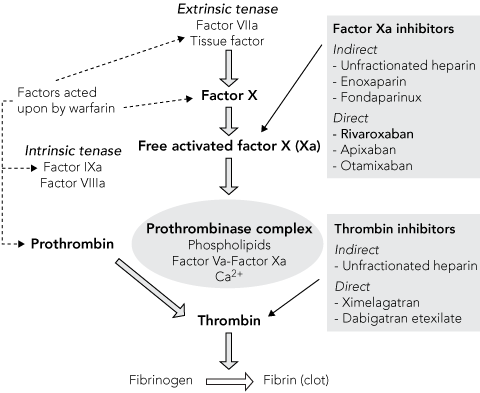
1 Mechanisms of action of new and current anticoagulant therapies within the coagulation cascade*
 | |||||||||||||||
|
* Adapted from Brighton TA.5 Printed with permission. | |||||||||||||||
2 Comparison of rivaroxaban with warfarin and enoxaparin
3 Summary of phase III studies of rivaroxaban for prophylaxis against venous thrombosis after major orthopaedic surgery
4 Drug profile summary of rivaroxaban
Dosage: Phase II and phase III clinical trials suggest that a dose of 10 mg is optimal for prophylaxis of thromboembolic disease.17,19 The treatment dose of 20 mg once daily is currently being evaluated in phase III treatment studies.
Administration: Once daily, orally.
Indications: It is anticipated that rivaroxaban will be marketed for indications similar to those approved for enoxaparin by the United States Food and Drug Administration, such as prophylaxis against thromboembolic disease in postsurgical patients.24 Other potential indications for rivaroxaban (eg, in atrial fibrillation, acute coronary syndromes and treatment of established thromboembolic disease) are currently being investigated.
Adverse effects: Few side effects of rivaroxaban have been reported in clinical trials to date. The most common adverse effects observed include headache, with some patients reporting nausea and vomiting (E310).20 Small numbers of patients in clinical trials showed elevated liver functions, but this was not dissimilar to those treated with enoxaparin.20,22,23
Contraindications and precautions: Clinical trials to date have excluded people with renal failure (as the drug is renally excreted). Prudent clinical practice would imply that precautions similar to those for warfarin should be applied to rivaroxaban: patients with active bleeding, intracerebral or gastrointestinal bleeding within the past 6 months, neurosurgery within the past 4 weeks, active peptic ulcer disease, a known bleeding disorder, prolonged prothombin time/international normalised ratio, pregnancy, thrombocytopaenia, impaired kidney function, or certain malignancies.14,15
Interactions: Current available evidence shows no significant drug interactions between rivaroxaban and non-steroidal anti-inflammatory drugs, aspirin, antacids, histamine H2-receptor antagonists, and digoxin.20-22 Evidence shows that rivaroxaban has interactions with potent cytochrome P450 3A4 inhibitors, such as ketaconazole, macrolides, and protease inhibitors.23
5 Important messages for patients
Rivaroxaban is a drug with anticlotting effects similar to warfarin and enoxaparin.
Unlike warfarin, rivaroxaban does not require frequent blood tests for monitoring.
Unlike enoxaparin, rivaroxaban does not require administration via injection.
Rivaroxaban has low potential for interaction with diet and other common medications.
Rivaroxaban may interact with some drugs, such as the antifungal drug ketaconazole, macrolide antibiotics (eg, clarithromycin, erythromycin, roxithromycin), or antiretroviral drugs (eg, atazanavir).
Rivaroxaban is not yet marketed in Australia.
- Abhishek K Verma1,2
- Timothy A Brighton3,2
- 1 Gosford Hospital, Gosford, NSW.
- 2 Faculty of Medicine, University of New South Wales, Sydney, NSW.
- 3 Prince of Wales Hospital, Sydney, NSW.
Timothy Brighton has received an honorarium from Bayer for an advisory role on Steering Committees for the EINSTEIN phase II and III clinical studies.
- 1. Campbell P, Roberts G, Eaton V, et al. Managing warfarin therapy in the community. Aust Prescr 2001; 24: 86-89.
- 2. Rang HP, Dale MM, Ritter JM. Pharmacology. 4th ed. New York: Churchill Livingstone Publishers, 2000: 316-318.
- 3. Warfarin Institute of America. Rivaroxaban (BAY 59-7939), Xarelto a possible replacement for warfarin. http://www.warfarinfo.com/rivaroxaban.htm (accessed Jun 2008).
- 4. Kalant H, Roschlau WHE. Principles of medical pharmacology. 6th ed. New York: Oxford University Press, 1998: 532-535.
- 5. Brighton TA. The direct thrombin inhibitor melagatran/ximelagatran. Med J Aust 2004; 181: 432-437. <MJA full text>
- 6. Kaul S, Diamond GA, Weintraub WS. Trials and tribulations of non-inferiority: the ximelagatran experience. J Am Coll Cardiol 2005; 46: 1986-1995.
- 7. Medscape. PETRO-Ex: Prevention of Embolic and Thrombotic Events in Patients with Persistent Atrial Fibrillation — Extension Trial. http://www.medscape.com/viewarticle/536379 (accessed Oct 2007).
- 8. Boehringer-Ingelheim. RELY Trial emergency information. Background data on dabigatran. https://www.rely-trial.com/RELYWeb/resources/jsp/emergency/dabigatran_bg.jsp (accessed Oct 2007).
- 9. Eriksson BI, Dahl OE, van Dijk CN, et al. A new oral anticoagulant, dabigatran etexilate, is effective and safe in preventing venous thromboembolism after total knee replacement surgery (the RE-MODEL Trial). Blood (ASH Annual Meeting Abstracts) 2006; 108: 573.
- 10. National Health and Medical Research Council. A guide to the development, evaluation and implementation of clinical practice guidelines. Canberra: NHMRC, 1999.
- 11. Turpie AG, Fisher WD, Bauer KA, et al. BAY 59-7939: an oral, direct factor Xa inhibitor for the prevention of venous thromboembolism in patients after total knee replacement. A phase II dose-ranging study. J Thromb Haemost 2005; 3: 2479-2486.
- 12. Gross PL, Weitz JI. New anticoagulants for treatment of venous thromboembolism. Arterioscler Thromb Vasc Biol 2008; 28: 380-386.
- 13. Laux V, Perzborn E, Kubitza D, Misselwitz F. Preclinical and clinical characteristics of rivaroxaban: a novel, oral, direct factor Xa inhibitor. Semin Thromb Hemost 2007; 33: 515-523.
- 14. Eriksson BI, Borris LC, Friedman RJ, et al; RECORD 1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008; 358: 2765-2775.
- 15. Kakkar AK, Brenner B, Dahl OE, et al; RECORD 2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled study. Lancet 2008; 372: 31-39.
- 16. Lassen MR, Ageno W, Borris LC, et al; RECORD 3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008; 358: 2776-2786.
- 17. Eriksson BI, Borris L, Dahl OE, et al; ODIXa-HIP Study Investigators. Oral, direct factor Xa inhibition with BAY 59-7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost 2006; 4: 121-128.
- 18. Agnelli G, Gallus A, Goldhaber SZ, et al; ODIXa-DVT Study Investigators. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59-7939 in Patients with Acute Symptomatic Deep-Vein Thrombosis) study. Circulation 2007; 116: 180-187.
- 19. Buller HR, Lensing AW, Prins MH, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging Study. Blood 2008; 112: 2242-2247.
- 20. Kubitza D, Becka M, Zuehlsdorf M, Mueck W. Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59-7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol 2006; 46: 549-558.
- 21. Kubitza D, Becka M, Mueck W, Zuehlsdorf M. Rivaroxaban (BAY 59-7939) — an oral, direct factor Xa inhibitor — has no clinically relevant interaction with naproxen. Br J Clin Pharmacol 2007; 63: 469-476.
- 22. Kubitza D, Becka M, Mueck W, Zuehlsdorf M. Safety, tolerability, pharmacodynamics, and pharmacokinetics of rivaroxaban — an oral, direct factor Xa inhibitor — are not affected by aspirin. J Clin Pharmacol 2006; 46: 981-990.
- 23. Hughes S. Rivaroxaban looks promising in DVT. heartwire 2007; 28 Jun. http://www.theheart.org/article/799303.do (accessed Jun 2008).
- 24. Hirsh J, O’Donnell M, Eikelboom JW. Beyond unfractionated heparin and warfarin: current and future advances. Circulation 2007; 116: 552-560.





Abstract
Warfarin and heparin are the traditional mainstay anticoagulant therapies for treating thromboembolic disease.
These drugs, with a documented history of utility, also have inherent difficulties in usage; in particular, the complicated monitoring and numerous drug–drug interactions of warfarin, and the need for parenteral administration of heparins.
New agents have recently emerged that target specific elements of the clotting pathway. Rivaroxaban, which inhibits activated factor X (Xa), is currently in clinical trials and is the most advanced factor Xa inhibitor.
The drug offers once-daily oral dosing, with no need for injections, dose titration, or frequent blood tests to monitor the international normalised ratio. It has a rapid onset of action and, although there is no specific antidote, it has a short plasma elimination half-life (about 5–9 hours).
Evidence from recently published large-scale phase III clinical trials shows rivaroxaban to be superior to enoxaparin for prophylaxis of venous thromboembolism after major orthopaedic surgery.
Studies have shown rivaroxaban to have a sound safety profile, with an incidence of bleeding similar to enoxaparin in phase III clinical trials.
Few side effects and drug–drug interactions between rivaroxaban and common medications have been found thus far, although some interactions with potent cytochrome P450 3A4 inhibitors have been observed.
It is hoped that rivaroxaban may be used as a first-line anticoagulant for prophylaxis of venous thromboembolic disease in postsurgical patients.