Indigenous Australians continue to live in conditions of enormous socioeconomic disadvantage and this is reflected in a life expectancy 17 years less than that of their non-Indigenous peers.1 Chronic diseases such as diabetes mellitus are thought to be the major cause of life-years lost for Indigenous people in the Northern Territory.2 However, these conditions may have less influence on life expectancy in central Australia, where infection-related mortality rates are higher than the rates in some African countries before the arrival of the current HIV pandemic.3
High background rates of infectious diseases among Indigenous Australians may underlie racial disparities in infection-related mortality in central Australia. Incidence rates of invasive pneumococcal disease4 and prevalence rates of bronchiectasis,5 for example, are among the highest in the world. The latter is associated with infection with the human T-cell lymphotropic virus type 1 (HTLV-1),5 which is endemic in central Australia but rare elsewhere.6 HTLV-1 infection predisposes to infection with the parasite Strongyloides stercoralis and to crusted scabies, providing portals of entry for enteric and gram-positive organisms, respectively.7,8 Deaths resulting from complicated strongyloidiasis have recently been reported at Alice Springs Hospital (ASH), in central Australia.7 Bloodstream infections (BSIs) continue to be associated with short-term mortality rates of 13%–21%,9-11 and 38% of infection-related deaths among Indigenous adults at ASH follow a BSI.3 The aim of our study was to characterise the pathogens responsible for BSI in central Australia and to determine the extent to which racial background influences BSI rates and mortality.
Our study was approved by the Central Australian Human Research Ethics Committee.
Between 2001 and 2005, 824 BSI episodes (753 in Indigenous and 71 in non-Indigenous patients) were recorded among 683 adults (614 Indigenous and 69 non-Indigenous) (Box 1). Health care-associated BSIs were uncommon in both groups, representing 45/485 episodes (9.3%) for Indigenous and 6/51 episodes (11.8%) for non-Indigenous patients. With the exception of malignancy and age, risk factors for sepsis were more common among Indigenous patients (Box 1).
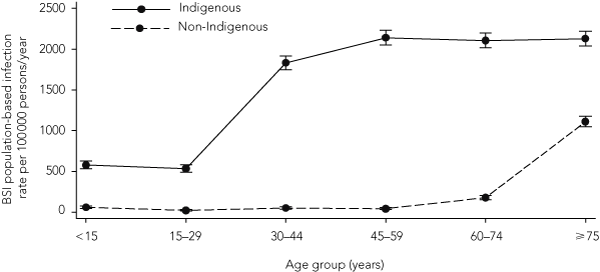
The admission-based BSI incidence rate between 2001 and 2005 was 26.5 (95% CI, 26.4–26.6) per 1000 adult admissions for Indigenous adults compared with 5.2 (95% CI, 5.1–5.2) per 1000 adult admissions for non-Indigenous adults (infection rate ratio [IRR], 5.13 [95% CI, 5.10–5.18]) (Box 2). Population-based BSI rates for the same period were 1354.7 (95% CI, 1256.3–1460.8) per 100 000 persons per year for Indigenous adults compared with 69.9 (95% CI, 55.1–88.6) per 100 000 persons per year for non-Indigenous adults (IRR, 19.4 [95% CI, 15.1–24.9]) (Box 2). Among Indigenous adults, population-based BSI rates increased sharply in the 30–44-years age group and remained relatively constant thereafter (Box 3).
The pathogens isolated from Indigenous and non-Indigenous adults are summarised in Box 4. Among Indigenous patients tested, HTLV-1 western blots were positive for 43.0% and S. stercoralis serology was positive for 35.4% (Box 1). Of 78 HTLV-1 seropositive Indigenous adults who were also tested serologically for S. stercoralis infection, 37 (47.4%) were seropositive.
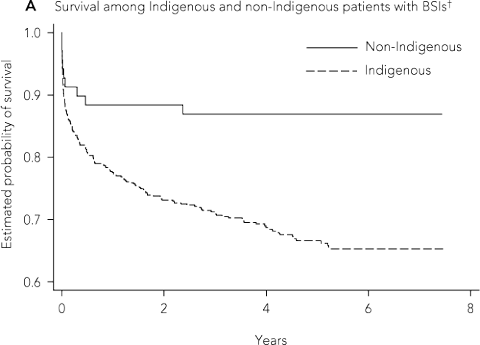
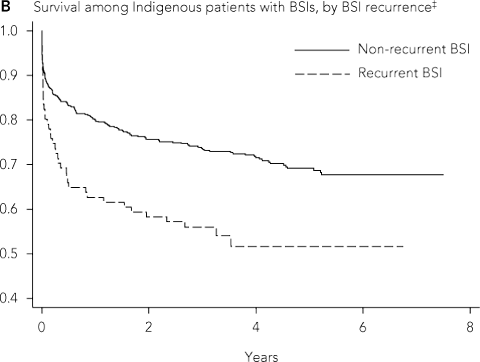
Among 614 Indigenous and 69 non-Indigenous adults there were 197 Indigenous and nine non-Indigenous deaths. For Indigenous patients, the mean age of death was 48.5 years (SD, 16.2 years), compared with 75.1 years (SD, 18.7 years) for non-Indigenous patients (P < 0.001). The risk of death during follow-up was 32.1% in Indigenous patients and 13.4% in non-Indigenous patients (hazard ratio [HR], 2.69 [95% CI, 1.38–5.25]; P = 0.004) (Box 5, A). Short-term mortality rates were similar between Indigenous and non-Indigenous adults: 7-day HR, 1.32 (95% CI, 0.48–3.69) (P = 0.59); 30-day HR, 1.47 (95% CI, 0.64–3.37) (P = 0.37). Risk of death was higher for Indigenous patients between 1 month after a BSI and the end of follow-up (HR, 5.16 [95% CI, 1.64–16.22]; P = 0.005). Indigenous adults were more likely than non-Indigenous adults to have at least two BSI episodes (16.5% v 5.0%; P = 0.019) (Box 1), and mortality among these patients was significantly higher than for Indigenous adults who had only a single episode (Box 5, B).
Our study reports one of the highest BSI incidence rates worldwide among a population of Indigenous adults residing in central Australia. Population-based estimates suggest that, each year over the period 2001–2005, nearly 2% of all Indigenous adults aged over 29 years suffered a life-threatening BSI. The combined admission-based cumulative incidence for Indigenous adults and children reported here (22.7/1000) approximate those for community-acquired BSI in Nigeria before the HIV pandemic (23.0/1000)12 and exceed those of hospitals in Thailand (9.5/1000)13 and Vietnam (20.4/per 1000).14 In contrast, admission-based (5.2/1000) and population-based (70/100 000 adult population per year) BSI rates for non-Indigenous adults were close to admission-based rates for adults with community-acquired BSI (4.7–5.4/1000)10,11,15,16 and consistent with population-based rates for all-cause sepsis (240–275/100 000 adult population per year)9,17 in other developed countries.
An increased susceptibility to infection as a result of comorbid conditions cannot fully account for the marked racial disparities in BSI rates in central Australia. Diabetes and alcohol dependence were the most frequent comorbidities among Indigenous adults, yet these are associated with only modest increases in the risk of invasive bacterial infection.18,19 Rates of diabetes mellitus among African Americans presenting with sepsis, for example, may approach those reported here,20 but this is associated with only a two-fold increase in sepsis risk relative to white Americans.9,17 In Australia, by contrast, profound differences exist between racial groups in their socioeconomic circumstances and resultant environmental exposure that might explain the markedly higher risk of BSI among Indigenous Australians. In some remote Indigenous communities the mean number of people living per house continues to be as high as 17;21 nearly half these houses do not have functioning sanitation, and opportunities to maintain skin hygiene are limited.22 In such environments, exposure to bacterial pathogens and S. stercoralis is likely, and HTLV-1 infection may further increase risk by predisposing people to complicated strongyloidiasis7 and crusted scabies.8
Previously, attempts to improve public health in remote Indigenous communities have largely focused on providing infrastructure.23 Inadequate housing and sanitation22 undoubtedly facilitate pathogen transmission in these communities, and further improvements are imperative. However, despite attempts to improve infrastructure, BSI rates for the Indigenous population of central Australia remain higher than those of some developing countries. The failure to reduce rates of bacterial infection in Indigenous communities may be comparable to the situation in developing countries in which the provision of water and sanitation alone does not improve health outcomes, owing to multiple routes of disease transmission, poor hygiene and a heavily contaminated environment.23,24 In such a setting, attempts to drive change externally by improving health infrastructure in isolation will fail.24 Consistent with the previous recommendations of the National Aboriginal Health Strategy,25 sustained improvement will require a coordinated approach23,24 based on dialogue, cultural understanding and the development of locally specific solutions by Indigenous people themselves.
1 Patient characteristics, outcomes and comorbid conditions for Indigenous and non-Indigenous adults admitted to Alice Springs Hospital with BSIs, 2001–2005
2 Infection rates and infection rate ratios for Indigenous and non-Indigenous patients admitted to Alice Springs Hospital with BSIs, 2001–2005
3 Population-based BSI incidence rates among Indigenous and non-Indigenous patients admitted to Alice Springs Hospital, by age, 2001–2005
4 Pathogens isolated from blood cultures taken from adult patients admitted to Alice Springs Hospital, 2001–2005*
- Lloyd J Einsiedel1
- Richard J Woodman2
- 1 Department of Medicine, Alice Springs Hospital, Alice Springs, NT.
- 2 Department of General Practice, Flinders University, Adelaide, SA.
We wish to thank Liselle Fernandes and Sheela Joseph for providing the comorbidity data. We received funding from the Northern Territory Rural Clinical School, an initiative of the Australian Department of Health and Ageing.
None identified.
- 1. Australian Institute of Health and Welfare and Australian Bureau of Statistics. The health and welfare of Australia’s Aboriginal and Torres Strait Islander peoples. Canberra: ABS, 2005. (AIHW Cat. No. IHW 14; ABS Cat. No. 4704.0.)
- 2. Zhao Y, Dempsey K. Causes of inequality in life expectancy between Indigenous and non-Indigenous people in the Northern Territory, 1981–2000: a decomposition analysis. Med J Aust 2006; 184: 490-494. <MJA full text>
- 3. Einsiedel LJ, Fernandes L, Woodman RJ. Racial disparities in infection-related mortality at Alice Springs Hospital, Central Australia, 2000–2005. Med J Aust 2008; 188: 568-571. <MJA full text>
- 4. Torzillo PJ, Hanna JN, Morey F, et al. Invasive pneumococcal disease in central Australia. Med J Aust 1995; 162: 182-186.
- 5. Steinfort DP, Brady S, Weisinger HS, Einsiedel L. Bronchiectasis in Central Australia: a young face to an old disease. Respir Med 2008; 102: 574-578.
- 6. Bastian I, Hinuma Y, Doherty RR. HTLV-1 among Northern Territory Aborigines. Med J Aust 1993; 159: 12-16.
- 7. Einsiedel L, Fernandes L. Strongyloides stercoralis: a cause of morbidity and mortality for indigenous people in Central Australia. Intern Med J 2008; 38: 697-703.
- 8. Hengge U, Currie BJ, Jager G, Schwartz RA. Scabies: a ubiquitous neglected skin disease. Lancet Infect Dis 2006; 6: 769-779.
- 9. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 2003; 348: 1546-1554.
- 10. Douglas MW, Lum G, Roy J, et al. Epidemiology of community-acquired and nosocomial bloodstream infections in tropical Australia: a 12-month prospective study. Trop Med Int Health 2004; 9: 795-804.
- 11. Geerdes HF, Ziegler D, Lode H, et al. Septicemia in 980 patients at a university hospital in Berlin: prospective studies during 4 selected years between 1979 and 1989. Clin Infect Dis 1992; 15: 991-1002.
- 12. Alausa KO, Montefiore D, Sogbetun AO, et al. Septicemia in the tropics. A prospective epidemiological study of 146 patients with a high case fatality rate. Scand J Infect Dis 1977; 9: 181-185.
- 13. Chierakul W, Rajanuwong A, Wuthiekanun V, et al. The changing pattern of bloodstream infections associated with the rise in HIV prevalence in northeastern Thailand. Trans R Soc Trop Med Hyg 2004; 98: 678-686.
- 14. Hoa NT, Diep TS, Wain J, et al. Community-acquired septicaemia in southern Viet Nam: the importance of multidrug-resistant Salmonella typhi. Trans R Soc Trop Med Hyg 1998; 92: 503-508.
- 15. Gosbell IB, Newton PJ, Sullivan EA. Survey of blood cultures from five community hospitals in south-western Sydney, Australia, 1993–1994. Aust N Z J Med 1999; 29: 684-692.
- 16. Brun-Buisson C, Doyon F, Carlet J. Bacteraemia and severe sepsis in adults: a multicenter prospective survey in ICUs and wards of 24 hospitals. Am J Crit Care Med 1996; 154: 617-624.
- 17. Esper AN, Moss M, Lewis CA, et al. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med 2006; 34: 2576-2582.
- 18. O’Brien JM, Lu B, Ali NA, et al. Alcohol dependence is independently associated with sepsis, septic shock and hospital mortality among adult intensive care unit patients. Crit Care Med 2007; 35: 345-350.
- 19. Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in diabetic patients. N Engl J Med 1999; 341: 1906-1912.
- 20. Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Occurrence and outcomes of sepsis: influence of race. Crit Care Med 2007; 35: 763-768.
- 21. McDonald MI, Towers RJ, Andrews RM, et al. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian Aboriginal communities where acute rheumatic fever is hyperendemic. Clin Infect Dis 2006; 43: 683-689.
- 22. Bailie RS, Stevens MR, McDonald E, et al. Skin infection, housing and social circumstances in children living in remote Indigenous communities: testing conceptual and methodological approaches. BMC Public Health 2005; 5: 128.
- 23. McDonald E, Bailie RS, Brewster D, Morris P. Are hygiene and public health interventions likely to improve outcomes for Australian Aboriginal children living in remote communities? A systematic review of the literature. BMC Public Health 2008; 8: 153-167.
- 24. Shuval HI, Tilden RL, Perry BH, Grosse RN. Effect of investments in water supply and sanitation on health status: a threshold saturation theory. Bull World Health Organ 1981; 59: 243-248.
- 25. Australian Department of Health and Aged Care. A National Aboriginal Health Strategy 1989. Canberra: Commonwealth of Australia, 1989 (reprinted 1996). http://www.health.gov.au/internet/main/publishing.nsf/Content/health-oatsih-pubs-NAHS1998 (accessed Apr 2010).





Abstract
Objective: To compare bloodstream infection (BSI) rates, pathogens and mortality among Indigenous and non-Indigenous adults in central Australia.
Design, participants and setting: Retrospective study of adult patients (aged ≥ 15 years) admitted to Alice Springs Hospital (ASH) between 1 January 2001 and 31 December 2005. Patients were followed up until 30 June 2008.
Main outcome measures: Admission-based and population-based BSI rates and mortality rates for Indigenous and non-Indigenous adults.
Results: During the study period, there were 824 BSI episodes (Indigenous, 753; non-Indigenous, 71). The admission-based BSI rate for Indigenous patients was 26.5 (95% CI, 26.4–26.6) per 1000 adult admissions, compared with 5.2 (95% CI, 5.1–5.2) per 1000 adult admissions for non-Indigenous patients (infection rate ratio [IRR], 5.13 [95% CI, 5.10–5.18]). The population-based BSI rate was 1354.7 (95% CI, 1256.3–1460.8) per 100 000 persons per year among Indigenous patients and 69.9 (95% CI, 55.1–88.6) per 100 000 persons per year among non-Indigenous patients (IRR, 19.4 [95% CI, 15.1–24.9]). These differences were not explained by higher comorbidity levels among Indigenous patients. Human T-cell lymphotropic virus type 1 and Strongyloides stercoralis infected 43% and 35%, respectively, of Indigenous patients tested. The risk of death during the follow-up period was 32.1% for Indigenous and 13.4% for non-Indigenous patients (hazard ratio [HR], 2.69 [95% CI, 1.38–5.25]; P = 0.004). Mortality rates were higher among Indigenous patients who had more than a single BSI (HR, 1.86 [95% CI, 1.32–2.62]; P < 0.001). The mean age at death was 48.5 years (SD, 16.2 years) for Indigenous patients and 75.1 years (SD, 18.7 years) for non-Indigenous patients (P < 0.001).
Conclusion: Indigenous adults living in central Australia experience BSI rates that are among the highest reported in the world. These are associated with a high risk of death, and are a likely consequence of the poor socioeconomic circumstances of Indigenous people.