Rates of acute lower respiratory infection (ALRI) are generally considered to reflect the ongoing and substantial health disparities between Indigenous and non-Indigenous children in Australia1 and other affluent countries such as the United States.2 Increasingly, early respiratory illness, especially in the first 2 years of life, is recognised as an important predeterminant of adult lung disease.3,4 Studies in Central Australia have shown an increased risk of chronic respiratory conditions, such as bronchiectasis, in Indigenous children, probably as a result of repeated respiratory infection in infancy.5,6
The morbidity of ALRI in NT Indigenous children has recently been analysed in a large study of pneumonia applying the WHO diagnostic protocol.7 Here, we report on a nested analysis within that study describing the epidemiology of ALRI in NT Indigenous children hospitalised in the first year of life.
Five public hospitals in the NT admit all NT Indigenous children requiring hospital treatment.
All primary and secondary diagnoses within an admission were included in the analysis.
All chest x-rays taken within any admission for any diagnosis were obtained from all hospital radiology departments across the NT. Films were read for WHO-defined pneumonia.8 According to the WHO protocol, x-ray films are read independently by two paediatric or respiratory physicians blinded to all data on the demographic, clinical and vaccination history of the subject. For admissions in which more than one x-ray was taken, any film deemed positive classified the admission episode as pneumonia. A positive first x-ray taken within 3 days of admission classified the admission episode as community-acquired pneumonia.
Incidence rates were calculated by dividing the number of cases by the person-time at risk from birth and are presented in units per 1000 child-years, with corresponding 95% confidence intervals. Relative risks and their exact 95% CIs were calculated to compare incidence rates by region and sex. The Kruskal–Wallis rank test was used to compare non-parametric distributions. Data were analysed using Stata, version 10.1 (StataCorp, College Station, Tex, USA).
There were 3227 (20%) episodes of care for ALRI, and 3626 diagnoses of ALRI for 2028 infants (21.8%); 665 (7.2%) had two or more episodes, and 55 (0.6%) had five or more. The incidence was 426.7 episodes per 1000 child-years (95% CI, 416.2–437.2). Incidence rates were 23% higher in boys (relative risk [RR], 1.23; 95% CI, 1.15–1.33) and over two times higher (RR, 2.12; 95% CI, 1.98–2.27) for infants in the Central Australian region of NT.
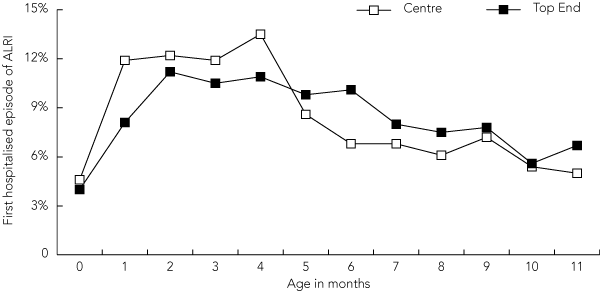
The median age at the time of first admission for ALRI was 4.6 months (IQR, 2.6–7.3) (Box 1). For children with multiple episodes, the median intervals between first to second, second to third, and third to fourth episodes were 54, 62 and 45 days, respectively. Intervals narrowed to medians of 26–33 days for subsequent episodes.
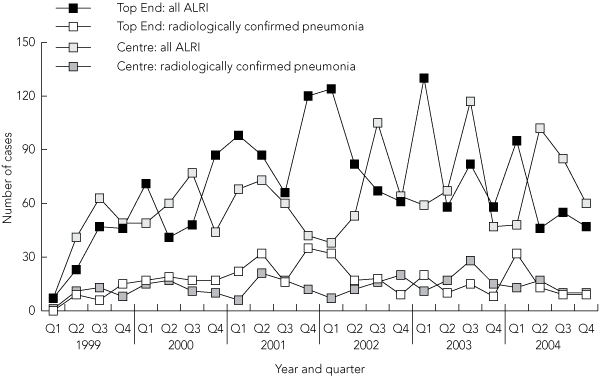
There were clear differences in temporal trends of ALRI diagnoses by NT region (Box 2). The associated diagnoses were dominated by seasonal peaks for bronchiolitis in the first quarter of the year in the Top End and in the third quarter of the year in the Central Australian region. Influenza outbreaks accounted for peaks apparent in the fourth quarter of 2000 and third quarter of 2003 in both regions. Seasonality was less clear for radiologically confirmed pneumonia.
One or more key comorbidities were present in 1445 (44.8%) of the 3227 episodes of care for ALRI: gastroenteritis in 832 (25.8%), anaemia in 896 (27.8%) and malnutrition in 128 (4.0%) episodes. Comorbidities were more frequent in ALRI episodes than in non-ALRI episodes and in pneumonia episodes than in bronchiolitis episodes (Box 3).
Of the ALRI diagnostic groups, bronchiolitis was the most common; rates of 352 per 1000 child-years (95% CI, 333–371) in the Central Australian region were double those of the Top End (RR, 1.99; 95% CI, 1.82–2.18). Discharge diagnoses in the 3227 episodes of care for ALRI and rates per 1000 child-years by diagnosis and NT region are presented in Box 4.
The rates of hospitalisation for ALRI (427 per 1000 child-years) among NT Indigenous infants are substantially higher than those in American Indian or Alaskan Native infants (116 per 1000 child-years),9 or those in developing countries (290 episodes per 1000 child-years in children aged < 5 years),10 as described in a meta-analysis of community-based studies. The incidence of WHO-defined radiologically confirmed pneumonia among children in the Central Australian region of NT (78.4 episodes per 1000 child-years; 95% CI, 68.1–89.6) is the highest incidence reported in published studies using the WHO protocol. These include studies of infants in the US,11 The Gambia,12 South Africa,13 Fiji,14 Uruguay,15 Indonesia,16 the Philippines,17 and Pakistan.18 Differences in case-ascertainment methods and access to hospital may partly explain the high rates we observed; however, they cannot entirely explain our findings.
Our study has identified a marked increase in hospital admissions for bronchiolitis (all causes) in infants. Between 1998 and 2000, the incidence in Central Australia was 190 per 1000 population aged under 12 months (88% were Aboriginal infants),19 compared with 352 per 1000 child-years in our study. Both studies reviewed all hospitalisations and included both primary and secondary diagnoses; however, the former study included the use of ICD-9-CM diagnoses, and incidence was calculated based on 1999 population data.19 A clear annual increase since 1999 was observed in our study, with a large epidemic in 2004. Hospitalisations for bronchiolitis have also been increasing in Western Australian Aboriginal infants,20 as well as in the American Indian population,9 for reasons that are not clear. The high proportion of radiologically confirmed pneumonia in bronchiolitis episodes is consistent with emerging evidence that viruses play an important role in the aetiology of pneumonia, independently and as interrelated agents in bacterial infections.21
The high proportion of infants admitted to hospital with ALRI and a comorbidity of anaemia, gastroenteritis and/or malnutrition, known risk factors for ALRI,22-24 is likely to be important. However, among studies using the WHO protocol, there is limited reporting of these conditions in populations without a high burden of HIV and malaria. It is unlikely that comorbidities alone are contributing to the high rates of disease in Indigenous infants. Overcrowding, young maternal age, low birthweight, and exposure to indoor and/or tobacco smoke are probable contributing factors. However, there is virtually no research specifically examining their relative importance for Indigenous children hospitalised with ALRIs. A small survey of 73 hospitalised Aboriginal children aged less than 14 years in Alice Springs found that the rate ratio for regular cough in children with household tobacco exposure was 2.77 (95% CI, 1.06–7.23).25 The Western Australian Aboriginal Child Health Survey found that parent-reported respiratory infections were more common in low-birthweight babies and those who had not been breastfed exclusively for 6 months.26
Our data provide no real clues as to the major aetiological causes of ALRI in Indigenous infants in the NT. The bulk of diagnoses are of unspecified aetiology. Our study also further highlights the limitations of using hospital discharge diagnoses alone to examine the epidemiology of disease at the population level, particularly given the suboptimal sensitivity for subcategories of ALRI.27-29 We specifically included all diagnoses, not just primary diagnosis codes, and all chest x-rays taken in any admission for any cause to maximise case ascertainment and to account for changes in coding practices over time. Furthermore, while there are concerns about the sensitivity and specificity of the WHO radiological definition of pneumonia,30 in our dataset 8.4% of radiologically confirmed cases had no corresponding ALRI diagnosis. Similarly, chest x-rays were not taken in 12% of infants with an ALRI diagnosis, and milder cases may have been missed.
The high rates of ALRIs and bronchiectasis in NT Indigenous infants warrant immediate attention. The Aboriginal health debate is currently dominated by the burden of the metabolic syndrome, chronic disease and substance abuse in adults. However, the huge burden of childhood ALRI has multiple consequences for the Aboriginal population. The response must be multipronged: research must continue, and policies that change the living environment and facilitate hygiene,31 improve educational outcomes for parents of the future, and enhance parenting skills32 must be a priority.
1 Frequency distribution (%) of the first episode of acute lower respiratory infection (ALRI) requiring hospitalisation in Northern Territory Indigenous children aged < 12 months, by age in months and NT region
2 Hospitalised episodes of acute lower respiratory infection (ALRI) and radiologically confirmed pneumonia in Northern Territory Indigenous children aged < 12 months, by time period and NT region
3 Number (%)* of episodes of care in hospital for acute lower respiratory infection (ALRI) with one or more key comorbidities, by ALRI diagnosis and Northern Territory region: Indigenous infants aged < 12 months, 1 January 1999 to 31 December 2004
* Percentages are of total ALRI episodes in each diagnosis category and region. |
|||||||||||||||
- Kerry-Ann F O’Grady1,2
- Paul J Torzillo3
- Anne B Chang1,4
- 1 Menzies School of Health Research, Charles Darwin University, Darwin, NT.
- 2 Centre for Clinical Research Excellence in Child and Adolescent Immunisation, Menzies School of Health Research and University of Melbourne, Darwin, NT.
- 3 Royal Prince Alfred Hospital, Sydney, and University of Sydney, Sydney, NSW.
- 4 Queensland Children’s Respiratory Centre, Queensland Children’s Medical Research Institute, Royal Children’s Hospital, Brisbane, QLD.
We would like to thank the PICTURE study team: Alan Ruben, Debbie Taylor-Thomson, Peter Morris, Grant Mackenzie, Paul Bauert, Gavin Wheaton, John DeCampo, Margaret DeCampo and Jane Benson. Kerry-Ann O’Grady is funded by a National Health and Medical Research Council (NHMRC) Postdoctoral Training Fellowship in Indigenous Health.
The study that led to this secondary analysis was funded by Wyeth Vaccines. Wyeth Vaccines had no role in the design, data collection, analysis and interpretation of the study, or in the writing of the article.
- 1. Li SQ, Guthridge S, d’Espaignet ET, Paterson B. From infancy to young adulthood: health status in the Northern Territory, 2006. Darwin: Northern Territory Department of Health and Families, 2007.
- 2. Brenneman G, Rhoades E, Chilton L. Forty years in partnership: the American Academy of Pediatrics and the Indian Health Service. Pediatrics 2006; 118: e1257-e1263.
- 3. Tennant PW, Gibson GJ, Pearce MS. Lifecourse predictors of adult respiratory function: results from the Newcastle Thousand Families Study. Thorax 2008; 63: 823-830.
- 4. Dharmage SC, Erbas B, Jarvis D, et al. Do childhood respiratory infections continue to influence adult respiratory morbidity? Eur Respir J 2009; 33: 237-244.
- 5. Valery PC, Torzillo PJ, Mulholland K, et al. Hospital-based case-control study of bronchiectasis in Indigenous children in Central Australia. Pediatr Infect Dis J 2004; 23: 902-908.
- 6. Chang AB, Masel JP, Boyce NC, Torzillo PJ. Respiratory morbidity in central Australian Aboriginal children with alveolar lobar abnormalities. Med J Aust 2003; 178: 490-494. <MJA full text>
- 7. O’Grady K, Taylor-Thomson D, Chang AB, et al. Rates of radiologically confirmed pneumonia as defined by the World Health Organization in Northern Territory Indigenous children. Med J Aust 2010; 192: 592-595. <eMJA full text>
- 8. World Health Organization Pneumonia Vaccine Trial Investigators Group. Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. Geneva: World Health Organization Department of Vaccines and Biologicals, 2001. http://www.who.int/vaccines-documents/DocsPDF01/www616.pdf (accessed Mar 2010).
- 9. Peck AJ, Holman RC, Curns AT, et al. Lower respiratory tract infections among American Indian and Alaska Native children and the general population of US children. Pediatr Infect Dis J 2005; 24: 342-351.
- 10. Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ 2004; 82: 895-903.
- 11. Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J 2006; 25: 779-781.
- 12. Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005; 365: 1139-1146.
- 13. Klugman KP, Madhi SA, Heubner R, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003; 349: 1341-1348.
- 14. Magree HC, Russell FM, Sa’aga R, et al. Chest x-ray confirmed pneumonia in children in Fiji. Bull World Health Organ 2005; 83: 427-434.
- 15. Hortal M, Estevan M, Iraola I, De Mucio B. A population-based assessment of the disease burden of consolidated pneumonia in hospitalized children under five years of age. Int J Infect Dis 2007; 11: 273-277.
- 16. Gessner BD, Sutanto A, Linehan M, et al. Incidences of vaccine-preventable Haemophilus influenzae type b pneumonia and meningitis in Indonesian children: hamlet-randomised vaccine-probe trial. Lancet 2005; 365: 43-52.
- 17. Lucero MG, Nohynek H, Williams G, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo-controlled trial. Pediatr Infect Dis J 2009; 28: 455-462.
- 18. Khan AJ, Hussain H, Omer SB, et al. High incidence of childhood pneumonia at high altitudes in Pakistan: a longitudinal cohort study. Bull World Health Organ 2009; 87: 193-199.
- 19. Bolisetty S, Wheaton G, Chang AB. Respiratory syncytial virus infection and immunoprophylaxis for selected high-risk children in Central Australia. Aust J Rural Health 2005; 13: 265-270.
- 20. Moore H, Burgner D, Carville K, et al. Diverging trends for lower respiratory infections in non-Aboriginal and Aboriginal children. J Paediatr Child Health 2007; 43: 451-457.
- 21. Rudan I, Boschi-Pinto C, Biloglav Z, et al. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008; 86: 408-416.
- 22. Schmidt WP, Cairncross S, Barreto ML, et al. Recent diarrhoeal illness and risk of lower respiratory infections in children under the age of 5 years. Int J Epidemiol 2009; 38: 766-772.
- 23. Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 2004; 80: 193-198.
- 24. Zaman K, Baqui AH, Yunus M, et al. Association between nutritional status, cell-mediated immune status and acute lower respiratory infections in Bangladeshi children. Eur J Clin Nutr 1996; 50: 309-314.
- 25. Hudson L, White A, Roseby R. Tobacco smoke exposure in hospitalised Aboriginal children in Central Australia. J Paediatr Child Health 2009; 45: 224-227.
- 26. Oddy WH, Kickett-Tucker C, De Maio J, et al. The association of infant feeding with parent-reported infections and hospitalisations in the West Australian Aboriginal Child Health Survey. Aust N Z J Public Health 2008; 32: 207-215.
- 27. Keren R, Wheeler A, Coffin SE, et al. ICD-9 codes for indentifying influenza hospitalizations in children. Emerg Infect Dis 2006; 12: 1603-1604.
- 28. van de Garde EM, Oosterheert JJ, Bonten M, et al. International classification of diseases codes showed modest sensitivity for detecting community-acquired pneumonia. J Clin Epidemiol 2007; 60: 834-838.
- 29. Guevara RE, Butler JC, Marston BJ, et al. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. Am J Epidemiol 1999; 149: 282-289.
- 30. Madhi SA, Klugman KP. World Health Organisation definition of “radiologically-confirmed pneumonia” may under-estimate the true public health value of conjugate pneumococcal vaccines. Vaccine 2007; 25: 2413-2419.
- 31. Torzillo PJ, Pholeros P, Rainow S, et al. The state of health hardware in Aboriginal communities in rural and remote Australia. Aust N Z J Public Health 2008; 32: 7-11.
- 32. Olds DL, Kitzman H, Hanks C, et al. Effects of nurse home visiting on maternal and child functioning: age-9 follow-up of a randomized trial. Pediatrics 2007; 120: e832-e845.





Abstract
Objective: To describe the epidemiology of acute lower respiratory infection (ALRI) and bronchiectasis in Northern Territory Indigenous infants hospitalised in the first year of life.
Design: A historical cohort study constructed from the NT Hospital Discharge Dataset and the NT Immunisation Register.
Participants and setting: All NT resident Indigenous infants, born 1 January 1999 to 31 December 2004, admitted to NT public hospitals and followed up to 12 months of age.
Main outcome measures: Incidence of ALRI and bronchiectasis (ICD-10-AM codes) and radiologically confirmed pneumonia (World Health Organization protocol).
Results: Data on 9295 infants, 8498 child-years of observation and 15 948 hospitalised episodes of care were analysed. ALRI incidence was 426.7 episodes per 1000 child-years (95% CI, 416.2–437.2). Incidence rates were two times higher (relative risk, 2.12; 95% CI, 1.98–2.27) for infants in Central Australia compared with those in the Top End. The median age at first admission for an ALRI was 4.6 months (interquartile range, 2.6–7.3). Bronchiolitis accounted for most of the disease burden, with a rate of 227 per 1000 child-years. The incidence of first diagnosis of bronchiectasis was 1.18 per 1000 child-years (95% CI, 0.60–2.16). One or more key comorbidities were present in 1445 of the 3227 (44.8%) episodes of care for ALRI.
Conclusions: Rates of ALRI and bronchiectasis in NT Indigenous infants are excessive, with early onset, frequent repeat episodes, and a high prevalence of comorbidities. These high rates of disease demand urgent attention.