Two children with advanced lung disease underwent successful cadaveric bilateral lobar lung transplantation, using lungs “cut down” from deceased adult donors — the first reported use of the technique in Australia. This approach, while it cannot address the lack of donor organs, may enable us to redress any size bias limiting paediatric lung transplantation.
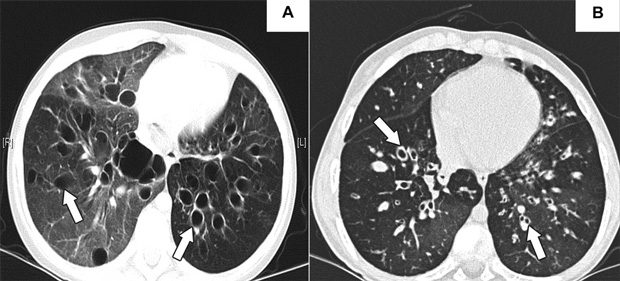
A previously healthy 9-year-old girl presented in early 2007 with an upper respiratory tract infection that progressed over 10 days to respiratory failure, requiring intubation and ventilation with high inspiratory pressures. Subsequent tracheal aspirates were positive for Mycoplasma (by polymerase chain reaction), with consistent serological results (antibody titres, 1 : 640). Computed tomography of the chest demonstrated widespread bronchiectasis (Box 1, A). Although she was extubated 6 weeks after initial presentation, she remained in hypercapnic respiratory failure (Pco2, 80 mmHg), requiring continuous oxygen supplementation (5 L/min) and bilevel non-invasive positive-pressure ventilation (BiPAP). She was listed for lung transplantation in May 2007, but, given the severity of her lung disease and in the absence of appropriately matched donor organs, the transplant team gave early consideration to cadaveric bilateral lobar transplantation using an adult “oversized” donor. This was performed in August 2007.
A 13-year-old girl with cystic fibrosis was referred for consideration of lung transplantation. She had been diagnosed with cystic fibrosis at birth (Δ508 homozygous, without liver, sinus or diabetic sequelae), and over the preceding 2 years developed progressive bronchiectasis (Box 1, B), necessitating supplemental oxygen and initiation of nocturnal BiPAP. She was initially listed for either lung transplantation or heart–lung transplantation; however, after 9 months of progressive respiratory failure (Pco2 increased to 46 mmHg; forced expiratory flow in 1 second [FEV1], 16%; forced vital capacity [FVC], 28% predicted), the transplant team considered cadaveric bilateral lobar transplantation, which was performed in September 2007.
Both children underwent cadaveric bilateral lobar transplantation as described by Starnes and colleagues1 for living-related lung transplantation. Briefly, the donor right lower lobe was resected, and the right upper and middle lobes were implanted, the anastomosis being performed at the right main bronchus. On the left, the inferior pulmonary vein, interlobar artery distal to its lingular branch, and bronchus were transected, and the lower lobe removed. Size mismatch was compensated for by seating the donor bronchus inside the recipient bronchus, while pulmonary vessel mismatch was taken up in the suture lines. Neither patient required cardiopulmonary bypass, and resected lobes were not used further.
Following surgery, both children were established on an internationally standardised immunosuppression regime, comprising prednisolone, tacrolimus and mycophenolate mofetil.2 Both patients made a good postoperative recovery, with short intensive care unit stays. Patient 1 required a longer inpatient stay for treatment of pneumonia. Neither patient developed allograft rejection, and lung function gradually improved (Box 2). Both patients were discharged to their respective tertiary hospitals for continuing follow-up. Both patients were well and without complication at follow-up 10 and 9 months postoperatively, respectively.
Lung transplantation is now an established treatment for patients with severe end-stage lung or pulmonary vascular disease. Despite attempts to increase organ donation worldwide, the number of patients requiring lung transplantation far exceeds the availability of donor lungs. In Australia, this is of particular concern for children awaiting appropriate size-matched donor organs.3 Review of the Australian and New Zealand Organ Donation Registry between 2002 and 2006 revealed that very few lungs are retrieved from paediatric donors younger than 14 years (26/497 lung donors).4
The number of children with severe lung disease warranting consideration of lung transplantation, both globally and in Australia, is, fortunately, very small. The most recent data from the International Society for Heart and Lung Transplantation show that only 65 paediatric lung transplantations were performed worldwide in 2005.2 However, of concern is that waiting-list mortality is greater for children than for adults — a worrying trend as fewer paediatric lung transplantations have been performed per annum, while adult lung transplantation numbers have increased.5
In Australia during 2006, 181 donor lungs were offered for lung transplantation, with only seven paediatric donors contributing, all of whom were aged 6–14 years (Ross Pettersson, Australian and New Zealand Cardiothoracic Organ Transplant Registry and Heart Transplant Data Manager, St Vincent’s Hospital, Sydney, NSW, personal communication). Despite an active policy of utilising “extended” donor organs (eg, from older donors or donors with previous cancer, smoking or aspiration history) wherever possible, only 30%–50% of available lungs are actually suitable for transplantation,6 further diminishing the number of available donor lungs, which is low by international standards.7 In the absence of appropriately size-matched organs, children from our institution have died while on the waiting list (2/9 listed in 2000–2007); after reviewing the 2007 donor referrals, it became apparent that the children described here would most likely have died while waiting.
Minimising paediatric waiting-list mortality requires consideration of non-traditional donor sources, such as live donors, who have been used in small numbers in the United States and Japan.8 The technique involves a bilateral lobar transplantation, typically taking one lobe from each of two larger, usually related, adult donors. Outcomes for living-donor bilateral lobar transplantation are similar to cadaveric lung transplantation, but there are significant ethical and technical issues with such an approach, and a potential 300% mortality rate. The number of these procedures being performed is declining.5 To our knowledge, no centre presently offers this service in Australia.
In adults, cadaveric lungs have been cut down to facilitate lung transplantation where size mismatch between donor and recipient could prevent transplant.9 Typically, this involves non-anatomical “lung shaving” or anatomical lobar resection. Rarely is this a bilateral extensive procedure, given the potential complications, including persistent air leaks, airway stenoses and stump dehiscence. Lobar transplantation is not specific to lung transplantation and has become common practice in liver transplantation; lessons may be learned from these experiences.10
Paediatric lobar transplantation has not been widely performed outside of the living-related scenario, but despite the additional surgical complexity, outcomes have proven comparable to cadaveric lung transplantation.8,9 Starnes and colleagues’ work suggests our two patients can be expected to ultimately achieve near-normal lung function,11 and their total lung capacity will increase as they grow.12
- 1. Starnes VA, Barr ML, Cohen RG. Lobar transplantation. Indications, technique, and outcome. J Thorac Cardiovasc Surg 1994; 108: 403-410; discussion 410-411.
- 2. Waltz DA, Boucek MM, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: ninth official pediatric lung and heart–lung transplantation report — 2006. J Heart Lung Transplant 2006; 25: 904-911.
- 3. Snell GI, Westall GP, Williams TJ. Lung transplantation: does age make a difference [editorial]? Med J Aust 2007; 187: 260-261. <MJA full text>
- 4. Australia and New Zealand Organ Donation Registry. The 2008 report. Data collected to December 2007. http://www.anzdata.org.au/ANZOD/ANZODReport/anzodreport.htm#2008 (accessed Feb 2008).
- 5. United States Organ Procurement and Transplantation Network 2006 Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Transplant Data 1996–2005. Rockville, Md: Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation. http://www.optn.org/AR2006/default.htm (accessed Feb 2008).
- 6. Snell GI, Griffiths A, Macfarlane L, et al. Maximizing thoracic organ transplant opportunities: the importance of efficient coordination. J Heart Lung Transplant 2000; 19: 401-407.
- 7. Opdam HI, Silvester W. Potential for organ donation in Victoria: an audit of hospital deaths. Med J Aust 2006; 185: 250-254. <MJA full text>
- 8. Date H, Yamane M, Toyooka S, et al. Current status and potential of living-donor lobar lung transplantation. Front Biosci 2008; 13: 1433-1439.
- 9. Aigner C, Mazhar S, Jaksch P, et al. Lobar transplantation, split lung transplantation and peripheral segmental resection — reliable procedures for downsizing donor lungs. Eur J Cardiothorac Surg 2004; 25: 179-183.
- 10. Rogiers X, Malago M, Gawad K, et al. In situ splitting of cadaveric livers. The ultimate expansion of a limited donor pool. Ann Surg 1996; 224: 331-339; discussion 339-341.
- 11. Starnes VA, Barr ML, Schenkel FA, et al. Experience with living-donor lobar transplantation for indications other than cystic fibrosis. J Thorac Cardiovasc Surg 1997; 114: 917-921; discussion 921-922.
- 12. Sritippayawan S, Keens TG, Horn MV, et al. Does lung growth occur when mature lobes are transplanted into children? Pediatr Transplant 2002; 6: 500-504.





None identified.