Iodine is an essential element for thyroid function, necessary for the normal growth, development and functioning of the brain and body. A diet deficient in iodine is associated with a wide spectrum of illness, collectively known as iodine deficiency disorders (IDD).1 While iodine deficiency is known to cause endemic goitre, its most deleterious effect may be on the developing brain of the fetus, ranging from mild brain dysfunction to irreversible intellectual impairment.2 It is the single most common cause of preventable mental retardation and brain damage in the world today.
In the past, iodine deficiency was a recognised health problem in Australia. There were geographic “hot spots” of goitre in mountainous areas of the Great Divide in eastern Australia, South Australia and Tasmania.3-5 Australia had remedied its iodine deficiency by the mid 1900s through a range of programs, including supplying iodised salt and iodine-supplemented bread, and population health messages. With the exception of Tasmania, Australia is now considered to be iodine sufficient. However, there is mounting evidence that, with the decline in salt intake and legislation no longer requiring iodine-supplementation of bread, there may be a re-emergence of iodine deficiency.6,7
A 1999 study found evidence of iodine deficiency in patients attending a Sydney teaching hospital.7 Occasional surveys of urinary iodine concentration (UIC) in small samples of people in metropolitan Sydney over the past 20 years by the Australian Centre for Control of Iodine Deficiency Disorders show a gradual but sustained decline in UIC.8,9 There has never been a systematic national survey to evaluate the iodine status of Australians.
In 2000, a Central Coast general practice reported a cluster of patients with goitre, the most obvious consequence of iodine deficiency, to the Central Coast Public Health Unit. Here, we report the prevalence of iodine deficiency in primary school children on the Central Coast by measuring urinary iodine levels and determining thyroid volumes as physiological measures of possible iodine deficiency, and comparing these with World Health Organization/International Council for the Control of Iodine Deficiency Disorders (WHO/ICCIDD) recommendations. This is the largest school-based survey using thyroid ultrasonography as a method of thyroid assessment in New South Wales.
Central Coast Health’s Public Health Unit and Westmead Hospital’s Department of Diabetes and Endocrinology conducted the study in November 2000. Central Coast Health’s Ethics Committee and the NSW Department of Education and Training approved the study.
The study design was a cross-sectional survey. With no existing prevalence data for the Central Coast, the choice of study population was based on a suspicion of iodine deficiency in a general practice population.10 Primary school children, as a group, are useful for assessing iodine deficiency, as they are easy to access and reflect current rather than remote iodine nutrition in the general population.10 A nearby primary school with a similar catchment to the general practice was selected. All children in Years 1–6 (age range, 5–13 years) were invited to participate. There were no exclusion criteria.
Parents were given an information sheet and consent form. The child’s sex and age were recorded. During the study, child health nurses spoke to teachers and some classes to promote participation.
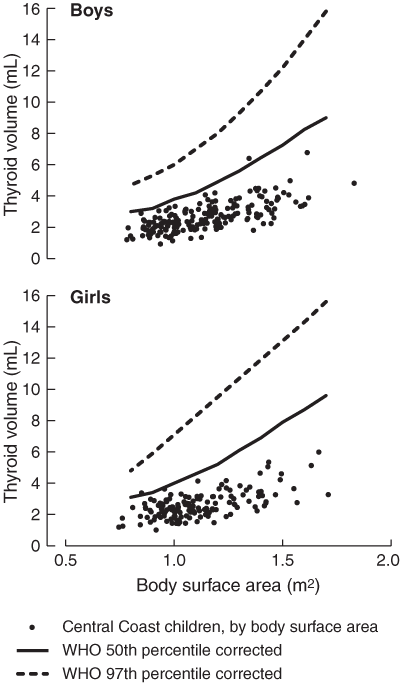
In each case, thyroid ultrasonography was performed by a single operator using a 7.5 MHz transducer with the child lying supine and the neck hyperextended. The volume of each lobe was calculated by the formula V (mL) = width × length × thickness × 0.479. The thyroid volume was the sum of the volumes of the two lobes. The volume of the isthmus was not included. Thyroid volumes greater than the 97th percentile are considered abnormally enlarged. Thyroid size was also examined by palpation.
Height and weight were measured by child health nurses using standardised procedures. The body surface area (BSA [m2]) was calculated by the formula BSA = W 0.425 × H 0.725 × 71.84 × 10 – 4, where W is the weight in kg and H the height in cm.
Each school class was asked to bring a first-morning sample of urine (which provides an adequate assessment of population-based UICs10,11) to school on a nominated day. Children were provided with a 70 mL urine sample jar labelled with their identification code, name and date. They were also given a sealed plastic bag for the sample and a brown paper bag to save embarrassment. Samples were collected from each class before the start of the school day.
Urine samples were kept on ice and delivered to Gosford Hospital’s patho-logy laboratory. Samples were frozen at –70°C and sent to the Institute of Clinical Pathology and Medical Research (ICPMR) laboratory at Westmead Hospital for analysis.
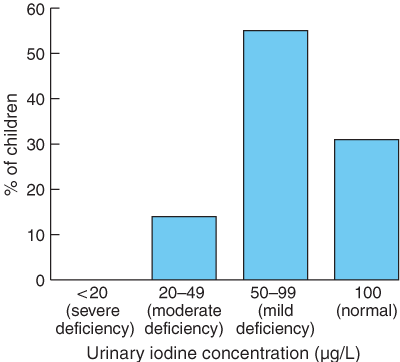
UIC was determined by the modified acid-digestion method. WHO/ICCIDD recommends that the median UIC for a population should be ≥ 100 μg of iodine per litre of urine. Mild iodine deficiency is a UIC in the range 50–99 μg/L; moderate iodine deficiency in the range 20–49 μg/L; and severe deficiency less than 20 μg/L. Not more than 20% of samples from a population should have a UIC below 50 μg/L.10,11
Urinary iodine data were analysed using an Excel spreadsheet and SAS statistical software.12,13 UIC is not considered normally distributed, and therefore the median is used as the measure of central tendency.10 Non-parametric variables were compared with the Wilcoxon rank sum test. Variables included age and sex.
Of the 465 children at the school, 324 (70%) underwent ultrasonography and palpation (180 boys and 144 girls). Box 1 shows thyroid volume for boys and girls after correcting for BSA. Applying WHO/ICCIDD reference values for thyroid volume gave a goitre prevalence of zero, using either age/sex-specific or BSA/sex-specific cut-off values. In girls, only four (3%) and one (1%) had thyroid volumes above WHO/ICCIDD median by age and BSA, respectively (P < 0.001). In boys, three (2%) and one (1%) had thyroid volumes above WHO/ICCIDD median by age and BSA, respectively (P < 0.001).
Of the 465 children at the school, 301 (65%) provided first-morning urine samples (168 boys and 133 girls). Participation rates ranged from 51% in Year 5 to 77% in Year 4. The median UIC for these years was 81 μg/L and 79 μg/L, respectively.
The median UIC for school children was 82 μg/L (interquartile range, 61–109 μg/L). Forty-two children (14%) had values below 50 μg/L. UIC was not related to age, class year or sex. Box 2 shows the distribution of UIC.
The school’s median UIC of 82 μg/L indicates mild iodine deficiency according to WHO/ICCIDD guidelines. This was similar to the median UIC of 84 μg/L previously recorded in primary school children in western Sydney.8,9 Both previous surveys reported that less than 20% of samples were below 50 μg/L (Central Coast, 14%; western Sydney, 13.8%).9 Our findings are consistent with evidence of mild to moderate iodine deficiency found in the 1999 Australian study, where median UICs ranged from 64 μg/L in volunteers to 104 mg/L in pregnant women (pregnant and lactating women require a daily intake of 150–200 μg/L).7 There is a direct relationship between UIC and iodine intake, with most iodine absorbed being excreted in the urine.11 Findings were also similar to a recent study involving 577 school children in Melbourne, Victoria, reporting a median UIC of 70 μg/L.14
All three independent studies sampling different areas of Sydney and the Central Coast of New South Wales report similar results.9 Surveys conducted in 1992 by the Australian Centre for Control of Iodine Deficiency Disorders showed a median UIC for Sydney residents of 180 μg/L.8 This suggests that iodine intake in mainland Australia has halved over a 10-year period. Studies in other developed countries, such as the United States and New Zealand, are also reporting a gradual decline in urinary iodine levels.15-17
The selection of samples and sample size are crucial for UIC to be a reliable marker.18 The recommended minimum sample size is 300 samples from a given population.11 Our study sample exceeds 300 and is the largest school-based iodine status survey using thyroid ultrasonography conducted in mainland Australia in recent times. The study was conducted in a medium-sized school, which, according to the 1996 Census Socio-Economic Index for Areas (SEIFA), is situated in an area ranked in the third quintile for relative socioeconomic disadvantage, and is immediately adjacent to one 4th- and several 1st- and 2nd-quintile areas.19 Thus, we believe the study’s data are representative of the Australian population.
Most parents were keen for their children to take part, but some children in Years 5 and 6 chose not to participate. Talking with the children revealed that some were too embarrassed to bring a urine sample to school — we feel this is unlikely to be correlated with UIC. However, we acknowledge that this drop in participation rates occurs at an age when the physiological demand for iodine is at its maximum.
The biochemical indicator of iodine status is a reasonably robust field measure. While individual iodine intake varies daily, this variation dampens out in population studies.10 UIC reflects recent intake, and so a population may appear iodine sufficient, and yet still be goitrous.
Ultrasonography is a fairly precise measurement of thyroid volume.10 However, it is noteworthy that thyroid size changes inversely in response to alterations in iodine intake, with a lag period of between 6 and 12 months in children.11 A combination of appropriate outcome measures such as UIC, thyroid volume and serum thyroid-stimulating hormone level will give a better picture of the long-term iodine status of a population.20
We agree with the authors of the 1999 Australian study that widespread, mild iodine deficiency is cause for concern, but would add the caveat to “look before we leap” to conclusions based on UIC.2 Despite their median UIC being in the mild iodine-deficiency range, the children in our study were not goitrous (shown by thyroid volume measurements in most of the children), even with the corrected WHO/ICCIDD data. This is consistent with previous findings for both UICs and relative lack of correlation with thyroid volume.14 Physiological outcome measures are essential to determine the impact of iodine deficiency in a community. For interpretation and proper assessment of thyroid volume measurements, local reference values (if available) are preferable, and thyroid size should be corrected for body surface area.
In conclusion, our report supports the growing body of evidence for the re-emergence of iodine deficiency in the Australian community. We join the call for a systematic national survey to determine iodine status by a combination of iodine-deficiency indicators such as UIC, goitre prevalence, thyroid volume and plasma thyroid hormone levels.
Received 26 February 2003, accepted 7 August 2003
- Kamala Guttikonda1
- Steven Boyages2
- Cheryl A Travers3
- Peter R Lewis4
- 1 Department of Diabetes and Endocrinology, Westmead Hospital, Westmead, NSW.
- 2 Central Coast Public Health Unit, Central Coast Health, Gosford, NSW.
We thank Dr Karen Byth and Mr Andrew Dixon for statistical analysis; Dr Lyndon Bauer, Dr Heather Reid (GPs); the Principal of the primary school, parents and children for their cooperation in the study; Central Coast Health’s Child Health Service, and Pathology Department; and the Institute of Clinical Pathology and Medical Research, Westmead Hospital; Central Coast Health’s Public Health Unit and Child Health Service for providing funds for data collection, analysis, interpretation and publication; Westmead Hospital’s Department of Diabetes and Endocrinology for contributing staff time and resources.
None identified.
- 1. Hetzel BS. Progress in the prevention and control of iodine deficiency disorders. Lancet 1987; 1: 266.
- 2. Boyages SC, Guttikonda K. Iodine status of Australia: look before we leap! [letter]. Med J Aust 2000; 172: 348.
- 3. Hetzel BS. The control of iodine deficiency. Med J Aust 1970; 2: 615-622.
- 4. Stewart JC, Vidor GI, Buttfield IH, Hetzel BS. Epidemic thyrotoxicosis in Northern Tasmania: studies in clinical features and iodine nutrition. Aust N Z J Med 1971; 3: 203-211.
- 5. Tasmanian Thyroid Advisory Committee. Study in disease surveillance, 1950–1979. Med J Aust 1981; 2: 234-238.
- 6. Burgess JR, Dwyer T, McArdle K, et al. The changing incidence and spectrum of thyroid carcinoma in Tasmania (1978–1998) during a transition from iodine sufficiency to iodine deficiency. J Clin Endocrinol Metabol 2000; 85: 1513-1517.
- 7. Gunton JE, Hams G, Fiegert M, McElduff A. Iodine deficiency in ambulatory participants at a Sydney teaching hospital: is Australia truly iodine replete? Med J Aust 1999; 171: 467-470. <eMJA full text>
- 8. Eastman CJ. Where has all our iodine gone? [editorial]. Med J Aust 1999; 171: 455-456. <eMJA full text>
- 9. Li M, Ma G, Guttikonda K, et al. Re-emergence of iodine deficiency in Australia. Asia Pacific J Clin Nutr 2001; 10: 200-203.
- 10. International Council for Control of Iodine Deficiency Disorders. Indicators for assessing IDD status. IDD Newsletter August 1999; 15: 33-38. Available at: www.people.virginia.edu/%7Ejtd/iccidd/newsletter/aug1999.htm (accessed Aug 2003).
- 11. World Health Organization Nutrition Unit. Indicators for assessing iodine deficiency disorders and their control through salt iodization. Document No. WHO/NUT/94.6. Geneva: WHO, 1994: 36.
- 12. Microsoft Excel 97 SR-2 [computer program]. Redmond, Wash: Microsoft Corporation, 1997.
- 13. SAS. Version 8.2 [computer program]. Cary, NC: SAS Institute Inc, 2002.
- 14. McDonnell CM, Harris M, Zacharin MR. Iodine deficiency and goitre in schoolchildren in Melbourne, 2001. Med J Aust 2003; 178: 159-162. <eMJA full text>
- 15. Hollowell JG, Staehling NW, Hannon WH, et al. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J Clin Endocrinol Metabol 1998; 83: 3401-3408.
- 16. Lee K, Bradley R, Dwyer J, Lee SL. Too much versus too little: the implications of current iodine intake in the United States. Nutr Rev 1999; 177-181.
- 17. Thomson CD, Colls AJ, Conaglen JV, et al. Iodine status of New Zealand residents as assessed by urinary iodine excretion and thyroid hormones. Br J Nutr 1997; 78: 901-912.
- 18. Dunn JT. Techniques for measuring urinary iodine — an update. IDD Newsletter November 1993; 9: 40-44. Available at: www.people.virginia.edu/%7Ejtd/iccidd/newsletter/idd1193.htm (accessed Apr 2001).
- 19. Census of population and housing. Socio-economic indexes for areas (SEIFA), New South Wales, Canberra: Australian Bureau of Statistics, Aug 1996. (ABS Catalogue no. 2033.1.30.001.)
- 20. International Council for Control of Iodine Deficiency Disorders. Standardization of ultrasound and urinary iodine determination for assessing iodine status: report of a technical consultation. IDD Newsletter May 2000; Volume 16, no. 2. Available at: www.people.virginia.edu/%7Ejtd/iccidd/newsletter/may2000.htm (accessed Aug 2003).





Abstract
Objective: To determine the prevalence of iodine deficiency in primary school children in an Australian urban population.
Design and setting: A cross-sectional survey of school children aged 5–13 years attending a public school on the Central Coast of New South Wales in November 2000.
Participants: 324 (70%) of the 465 children enrolled in the school (180 boys; 144 girls).
Main outcome measures: Thyroid volumes compared with World Health Organization/International Council for the Control of Iodine Deficiency Disorders (WHO/ICCIDD) thyroid volume reference values. Iodine status based on WHO/ICCIDD urinary iodine concentration (UIC) categories (normal, ≥ 100 μg per litre of urine [μg/L]; mild iodine deficiency, 50–99 μg/L; moderate deficiency, 20–49 μg/L; severe deficiency, < 20 μg/L); not more than 20% of the population should have a UIC below 50 μg/L.
Results: Median UIC for school children was 82 μg/L, and 14% of children had UICs below 50 μg/L. Thyroid volume reference values indicated a prevalence of goitre of zero. In girls, only four (3%) and one (1%) had thyroid volumes above the WHO/ICCIDD medians by age and body surface area (BSA), respectively (P < 0.001). In boys, three (2%) and one (1%) had thyroid volumes above WHO/ICCIDD medians by age and BSA, respectively (P < 0.001).
Conclusion: Despite the median UIC being less than ideal, most children were not goitrous. This underscores the importance of using physiological outcome measures in areas where iodine deficiency is marginal before concluding the need for iodine supplementation based purely on median UIC. We call for a systematic national survey to determine iodine status using a combination of iodine deficiency indicators.