Iodine is an essential element for the production of the thyroid hormones, triiodothyronine (T3) and thyroxine (T4). A woman needs more iodine during pregnancy to maintain normal metabolism as well as meet the requirements of T4 and iodide transfer to the fetus. An insufficient supply of thyroid hormones to the developing brain of the fetus can result in congenital anomalies and intellectual impairment.1
We used two indicators of iodine status: maternal urinary iodine concentration (UIC) and newborn whole-blood thyroid stimulating hormone (TSH) level. UIC is a good indicator of a previous day’s dietary iodine intake, as over 90% of iodine absorbed is eventually excreted in the urine. While individual iodine intake varies daily, UIC is a reasonable population indicator of iodine status.2 The newborn thyroid has limited iodine stores, and even mild deficiency will increase TSH secretion in the blood. In epidemiological studies, populations with 3% of newborns with TSH levels > 5 mIU/L whole-blood are considered at risk of iodine deficiency.3
Few studies have examined the possible correlation between maternal UIC and newborn TSH level. McElduff and colleagues in 2002 reported that they had not found the expected relationship between maternal UIC and neonatal TSH level.4 This prompted us to investigate the correlation between these two indicators to confirm these findings.
Women consented to provide a urine sample and gave permission for us to access their newborn’s TSH value, routinely collected by the NSW Newborn Screening Programme. Women from non-English speaking countries were included in the study; however, they comprised fewer than 5% of the women attending local antenatal care services.5
Participating women provided a spot urine sample. Urine samples were kept on ice and delivered to the pathology laboratory at Gosford Hospital. Samples were frozen at − 70°C and sent to the Institute of Clinical Pathology and Medical Research laboratory at Westmead Hospital for analysis. UIC was determined by the modified acid-digestion method.6
Newborn screening tests are offered to all babies born in NSW. A heel-prick blood spot sample is collected onto pre-printed filter paper cards usually at 48–72 hours after birth. Screening before 48 hours produces a high rate of false positive results due to a TSH surge immediately after birth.4 The 1235 AutoDELFIA automatic fluoroimmunoassay system (Wallac/Perkin Elmer Life Sciences, Turku, Finland) is used for determining newborn whole-blood TSH level. The TSH assay can discriminate to within 2 mIU/L blood. Time of birth (public hospitals only) was collected and time of heel-prick test estimated to determine the effect on TSH values of the age of the newborn when the heel-prick sample was taken.
Newborns shown to have congenital hypothyroidism and pregnant women taking thyroxine were excluded.
Box 1 gives the indicators for assessing iodine status based on the guidelines of the World Health Organization and the International Council for the Control of Iodine Deficiency Disorders (WHO/ICCIDD). These guidelines recommend that not more than 20% of urine samples in a population should have iodine levels < 50 μg/L and not more than 3% of neonates should have whole-blood TSH values > 5 mIU/L.
The denominator population was estimated from mean confinement rates for the Central Coast over a 3-year period, and attendance sheets at private clinics.7 This gave a response rate of 81% for women giving birth at the public hospitals and 77% for women attending the three obstetrician clinics.
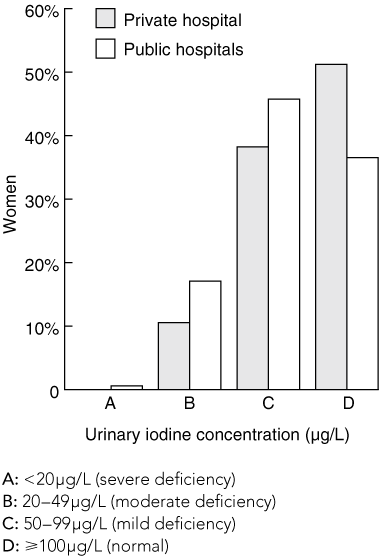
Of 815 women, 796 (97.7%) provided a urine sample. Their mean age was 28.8 years (SD, 5.8). The median UIC was 85 μg/L, with a range of 19–1510 μg/L (interquartile range, 58 μg/L). There were 132 (16.6%) women with a UIC < 50 μg/L. The median UIC for women giving birth at the public hospitals was 82 μg/L, and it was 101 μg/L for women giving birth at the private hospital; 36.6% (246/673) of women giving birth at the public hospitals had a UIC of 100 μg/L or more, compared with 51.2% (63/123) of women at the private hospital (χ2 = 9.42; df = 1; P = 0.002) (Box 2). The natural log of UIC for women giving birth at the private hospital (mean, 4.58; CI 95%, 4.48–4.68) was significantly higher than that for women giving birth at the public hospitals (mean, 4.40; CI 95%, 4.36–4.44) (t = 3.35; df = 794; P = 0.0008).
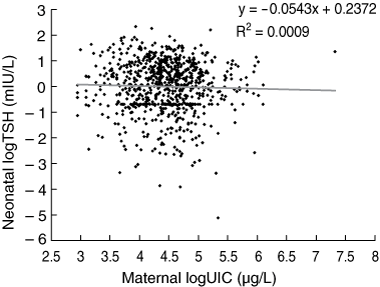
A weak negative association was observed between 797 matched-pairs of neonatal TSH levels and maternal UIC. No statistically significant linear correlation was found between the natural log of neonatal TSH and the natural log of maternal UIC (r = − 0.03; P = 0.4) (Box 3).
The median UIC for women was 85 μg/L, indicating mild iodine deficiency. Almost 17% of pregnant women had a UIC < 50 μg/L (not more than 20% should be < 50 μg/L).3 Most newborns appeared to be receiving sufficient iodine, with only 2.2% having TSH values > 5 mIU/L. There was a weak negative correlation between the natural log of neonatal TSH and the natural log of maternal UIC, but this did not reach statistical significance.
To date, our study is the largest of its kind in Australia. It included public and private hospital settings, thus providing a reasonably representative sample of the target group. Our finding of mild iodine deficiency in pregnant women and iodine sufficiency in their newborns highlights the value of using two indicators of iodine status.3
There are several reasons for caution in interpreting neonatal TSH levels. These include the sensitivity of the assay used and the surge in TSH level related to the stress of birth.3,4,8 Further, the method used to establish the criteria for neonatal TSH level was not specified in the WHO guidelines. The use of different methods (eg, radioimmunoassay or fluoroimmunoassay) gives small systematic differences in results obtained, enough to influence the proportion of results above a cutoff (eg, 3 mIU/L).9
Furthermore, neonatal TSH concentration is dependent on the time of heel-prick sample after birth.3,8 The WHO states that, for epidemiological studies, heel-prick blood specimens should be collected 72 hours after birth, to avoid the surge in TSH level.3 Most of our specimens were collected between 48 and 72 hours after birth, which may have affected our ability to detect a correlation between newborn TSH level and maternal UIC. A meaningful correlation may not have been found because TSH level was not adjusted for neonatal age (in hours) at the time of sampling, and time of birth was only available for babies born in the public hospitals.
The lack of a statistically significant linear correlation between newborn TSH level and maternal UIC was also found in other Australian studies.4,10 McElduff and colleagues reported a positive correlation for a small sample of 84 mothers and babies, but this was not statistically significant.
We found a statistically significant difference between the median UIC for women giving birth at the public hospitals (82 μg/L) and the private hospitals (101 μg/L). A Tasmanian study found that children enrolled in a private school had a higher UIC than children from public schools; however, the difference was not statistically significant.11 Anecdotal evidence suggests that women giving birth at private hospitals may be more likely to seek information, education and planning on their intended pregnancy and to take supplements, some containing iodine.
Studies on the dietary intake of pregnant women indicate that those from lower income and education levels tend to have poorer diets, and supplementation is highest among those with higher education.12-15 A greater frequency of fish consumption has been reported with higher levels of education (saltwater fish being rich in iodine).16 Regardless, the median UIC in women giving birth at both public and private hospitals was well below the level of optimal iodine nutrition during pregnancy.1
Despite the TSH values being within the normal range, it is clear that pregnant women are not getting adequate dietary iodine. The WHO/ICCIDD recommendations used for assessing urinary iodine status are based on the general population. Recently, the ICCIDD determined that the median urinary iodine level for optimal iodine nutrition during pregnancy should be in the range 150–230 μg/L daily.1 In our study, only 12% of pregnant women had a UIC of 150 μg/L or more. Similar findings of low iodine status in different population groups have been reported in other Australian studies.4,6,11,17,18
We have found iodine deficiency among a population by measuring UIC, yet this deficiency does not appear to be reflected in other indicators of iodine status — in this case, neonatal TSH level. Similarly, our study of iodine deficiency in primary school children in 2000 reported that, despite the children being mildly iodine deficient, none were goitrous (the most obvious consequence of iodine deficiency).17 This raises the question of what is the most appropriate indicator for assessing iodine status in the general population.
For example, iodine deficiency in pregnant women may be better detected using maternal free T4 as an indicator in the first trimester. A small decrease in serum free T4 during pregnancy is an important risk factor for impaired psychomotor development in infants.19,20 One area for future research lies in the use of TSH and T4 in pregnancy as screening tools for epidemiological studies. This would require the development of method-specific, trimester-specific, reference intervals for free T4 estimates in pregnancy.21 It would be of value to correlate maternal TSH and T4 in each trimester with psychomotor and mental development in the first year of life.20
Finally, this study highlights the need for ongoing surveillance of the iodine status of NSW communities to determine trends over time and health outcomes associated with mild iodine deficiency.2,4 We support the call to raise awareness of the need for pregnant and lactating women to increase their iodine intake.18 Our data add to the pool of national data being collected by the Australian Centre for Control of Iodine Deficiency Disorders to inform future research and potential intervention strategies in Australia.
Received 2 February 2006, accepted 12 April 2006
- Cheryl A Travers1
- Kamala Guttikonda2
- Carol A Norton1
- Peter R Lewis1
- Lyndall J Mollart3
- Veronica Wiley4
- Bridget Wilcken4
- Creswell J Eastman5
- Steven C Boyages6
- 1 Central Coast Public Health Unit, Northern Sydney Central Coast Area Health Service, Gosford, NSW.
- 2 Private Clinic, Gosford, NSW.
- 3 Maternity Services, Central Coast Health, Gosford, NSW.
- 4 NSW Newborn Screening Programme, The Children’s Hospital at Westmead, Sydney, NSW.
- 5 International Council for the Control of Iodine Deficiency Disorders (ICCIDD), Asia Pacific Region, Sydney, NSW.
- 6 Area Executive Unit, Sydney West Area Health Service, Sydney, NSW.
We thank the other iodine team members: Carol McCloy (Divisional Manager, Women’s Children and Family Health), Glenda Gilmore (Nurse Unit Manager Night Duty, Maternity Services), Andrew Dixon (statistical analysis), Nicola Cooper (Project Officer) and Paul Cook (data management). We extend our appreciation to the midwives of Central Coast Health, obstetricians and their staff, participating women, staff at Gosford Hospital’s pathology laboratory, staff of the NSW Newborn Screening Programme, and Dr Gary Ma (Principal Scientist, Institute of Clinical Pathology and Medical Research) and staff.
None identified.
- 1. Delange F. Optimal iodine nutrition during pregnancy, lactation and the neonatal period. Int J Endocrinol Metab 2004; 2: 1-12.
- 2. International Council for Control of Iodine Deficiency Disorders. Indicators for assessing IDD status. IDD Newsletter Aug 1999; 15: 33-38.
- 3. World Health Organization. Indicators for assessing iodine deficiency disorders and their control through salt iodization. Geneva: WHO, 1994: 36. (Document No. WHO/NUT/94.6.)
- 4. McElduff A, McElduff P, Gunton JE, et al. Neonatal thyroid-stimulating hormone concentrations in northern Sydney: further indications of mild iodine deficiency? Med J Aust 2002; 176: 317-320. <MJA full text>
- 5. Centre for Epidemiology and Research, NSW Department of Health. New South Wales mothers and babies 2002 report. Available at: http://www.health.nsw.gov.au/public-health/mdc/mdcrep02.html (accessed May 2006).
- 6. Li M, Ma G, Guttikonda K, et al. Re-emergence of iodine deficiency in Australia. Asia Pac J Clin Nutr 2001; 10: 200-203.
- 7. Centre for Epidemiology and Research. NSW Department of Health. New South Wales mothers and babies 2001 report; 2002 report; 2003 report. Available at: http://www.health.nsw.gov.au/pubs/subs/sub_maternal.html (accessed May 2006).
- 8. Copeland DL, Sullivan KM, Houston R, et al. Comparison of neonatal thyroid-stimulating hormone levels and indicators of iodine deficiency in school children. Public Health Nutr 2002; 5: 81-87.
- 9. Gruñeiro-Papendieck L, Chiesa A, Mendez V, et al. Neonatal TSH levels as an index of iodine sufficiency: differences related to time of screening sampling and methodology. Horm Res 2004; 62: 272-276.
- 10. Chan SS, Hams G, Wiley V, et al. Postpartum maternal iodine status and the relationship to neonatal thyroid function. Thyroid 2003; 13: 873-876.
- 11. Hynes KL, Blizzard CL, Venn AJ, Dwyer T. Persistent iodine deficiency in a cohort of Tasmanian school children: associations with socio-economic status, geographical location and dietary factors. Aust N Z J Public Health 2004; 28: 476-481.
- 12. Daltveit AK, Vollset SE, Lande B, Oien H. Changes in knowledge and attitudes of folate, and use of dietary supplements among women of reproductive age in Norway 1998-2000. Scand J Public Health 2004; 32: 264-271.
- 13. Goldberg BB, Alvarado S, Chavez C, et al. Prevalence of periconceptional folic acid use and perceived barriers to the postgestation continuance of supplemental folic acid: survey results from a Teratogen Information Service. Birth Defects Res A Clin Mol Teratol 2006; 76: 193-199.
- 14. Kaim I, Penar A, Sochacka-Tatara E, et al. [Intake of pharmacologic supplements of vitamins and minerals during pregnancy. Survey conducted in Krakow] [Polish]. Przegl Lek 2004; 61: 776-779.
- 15. McGovern E, Moss H, Grewal G, et al. Factors affecting the use of folic acid supplements in pregnant women in Glasgow. Br J Gen Pract 1997; 47: 635-637.
- 16. Moreira PA, Padrao PD. Educational and economic determinants of food intake in Portuguese adults: a cross-sectional survey. BMC Public Health 2004; 4: 58. Available at: http://www.biomedcentral.com/1471-2458/4/58 (accessed Jan 2006).
- 17. Guttikonda K, Travers C, Lewis P, Boyages S. Iodine deficiency in urban school children: a cross-sectional analysis. Med J Aust 2003; 179: 346-348. <MJA full text>
- 18. Li M, Eastman CJ, Waite KV, et al. Are Australian children iodine deficient? Results of the Australian National Iodine Nutrition Study. Med J Aust 2006; 184: 165-169. <MJA full text>
- 19. Morreale de Escobar G, Obregon MJ, Escobar del Rey F. Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia. J Clin Endocrinol Metab 2000; 85: 3975-3987.
- 20. Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf) 1999; 50: 149-155.
- 21. Mandel SJ, Spencer CA, Hollowell JG. Are detection and treatment of thyroid insufficiency in pregnancy feasible? Thyroid 2005; 15: 44-53.





Abstract
Objectives: To determine whether pregnant women and their newborns show evidence of iodine deficiency, and to examine the correlation between maternal urine iodine concentration (UIC) and newborn thyroid-stimulating hormone (TSH) level.
Design: A cross-sectional study.
Setting: Hospital antenatal care services (March–May 2004) and private obstetrician clinics (June 2004) in the Central Coast area of New South Wales.
Participants: 815 pregnant women (≥ 28 weeks’ gestation) and 824 newborns.
Main outcome measures: World Health Organization/International Council for the Control of Iodine Deficiency Disorders criteria for assessing severity of iodine deficiency (recommended levels: < 20% of urine samples in a population with UIC < 50 μg/L; and < 3% of newborns with whole-blood TSH level > 5 mIU/L).
Results: The median UIC for pregnant women was 85 μg/L, indicating mild iodine deficiency. Almost 17% of pregnant women had a UIC < 50 μg/L, and 18 newborns (2.2%) had TSH values > 5 mIU/L. There was no statistically significant linear correlation between neonatal whole-blood TSH level and maternal UIC (r = − 0.03; P = 0.4). Mothers with a UIC < 50 μg/L were 2.6 times (relative risk = 2.65; 95% CI, 1.49–4.73; P = 0.01) more likely to have a baby with a TSH level > 5 mIU/L.
Conclusion: The pregnant women surveyed were mildly iodine deficient. TSH values for their newborns were mostly within acceptable limits. Ongoing surveillance of the iodine status of NSW communities to establish trends over time is recommended.