Inappropriate antibiotic use is a common problem in Australia and elsewhere and is associated with increasing emergence of antibiotic-resistant pathogens and increasing healthcare costs.1-3 Despite evidence-based guidelines to support rational antibiotic use, practical control of prescribing has proven difficult.2-5 One method used by some hospitals has been to develop a list of restricted antibiotics, which, because of their high cost (either acquisition cost or cost related to the emergence of antibiotic resistance), cannot be prescribed without justification and without obtaining an approval number from the institution’s infectious diseases department.
At our institution (Austin Health, a tertiary hospital in Melbourne), a phone-based system for obtaining approval has proven effective in controlling the appropriate use of at least third-generation cephalosporins and glycopeptides. But its implementation has come at a price.4,5 In a recent audit we found that our Infectious Diseases Department could expect to handle 3640 approval phone calls per year (8–14 per day), which, at 2 minutes per phone call, constituted the equivalent of about 3 weeks a year of a full-time staff member’s time spent entirely on the telephone (unpublished data). Not surprisingly, this system risks having a negative impact on other aspects of infectious disease healthcare provision, as well as our registrar program.
To address these issues, we developed an infectious diseases electronic antibiotic advice and approval system (“IDEA3S”). The purpose of IDEA3S was to provide quick and accessible computer-generated antibiotic approval for common evidence-based indications and reduce the number of phone-based approvals while maintaining current levels of appropriate antibiotic prescribing.
To identify the antibiotics for which approvals were most commonly requested at Austin Health, infectious disease registrars recorded all phone-based antibiotic approvals during an 8-week period in late 2001. A working party of infectious disease physicians, pharmacists and members of the Respiratory Medicine, Surgery and Emergency departments decided which agents and indications should be included in IDEA3S. Decisions were based on the frequency of approval requests, the availability of evidence-based prescribing data for specified indications, and the potential impact of inappropriate use.
IDEA3S was designed to have the following features:
For community-acquired pneumonia it uses pneumonia severity, as defined by the Pneumonia Severity Index (PSI), to guide empirical antibiotic therapy.6,7 In addition to providing a rapid electronic PSI calculator, IDEA3S stores the entered data and PSI score for later analysis;
For acute infective exacerbations of chronic obstructive pulmonary disease, IDEA3S uses an evidence-based approach to guide therapy.8
IDEA3S was developed to be tightly integrated with the hospital’s pharmacy dispensing system (“Merlin”, Pharmhos Software Pty Ltd, Port Melbourne, Australia) and to be accessible via the hospital’s computer network.
The system was introduced in March 2002. Post-implementation findings were audited 12 and 18 months later and compared with pre-implementation data.
The impact of IDEA3S was assessed as follows:
The number of “hits” on IDEA3S (both approvals and non-approvals) were stored automatically by IDEA3S and audited monthly.
The use of ceftriaxone/cefotaxime and vancomycin (the two antibiotics for which approvals were most frequently sought) was monitored, with usage data standardised as defined daily doses (DDDs) per 1000 patient bed-days.9
The number of approved and non-approved courses of ceftriaxone/cefotaxime and vancomycin were assessed during the 12 months before the introduction of IDEA3S and the 18 months after its introduction (both IDEA3S-based and phone-based approvals were counted).
Among patients with community-acquired pneumonia for whom approval for the use of ceftriaxone/cefotaxime was sought from IDEA3S, concordance between the antibiotic regimen recommended by IDEA3S and the actual combination used was assessed over a 9-month period using the following definitions: “exact concordance” — regimen recommended by IDEA3S followed exactly; “concordance” — IDEA3S recommendations followed except for the use of oral roxithromycin or doxycycline instead of intravenous erythromycin (or vice versa); “non-concordance” — ceftriaxone/cefotaxime used contrary to IDEA3S recommendations, or no treatment given for “atypical” pathogens (Mycoplasma, Legionella or Chlamydia spp.).
Implementation and ongoing problems associated with the use of IDEA3S were assessed and summarised during education and feedback sessions with hospital medical officers, senior medical staff, pharmacists and ward staff.
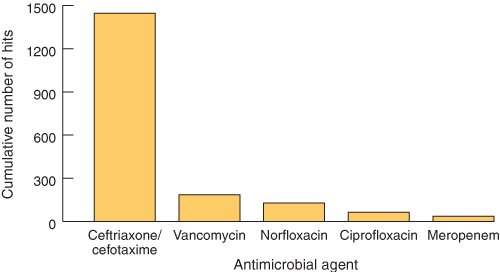
Requests for approval to use ceftriaxone/cefotaxime or vancomycin constituted 83% of all IDEA3S “hits” during the initial 18 months of its operation (Box 1). The number of ceftriaxone/cefotaxime and vancomycin courses for which approval was sought, before and after the introduction of IDEA3S, is shown in Box 2. After March 2002, IDEA3S-based approval for these agents constituted 48% of all approvals (ie, phone-based approvals were reduced by about half). Data on ceftriaxone/cefotaxime and vancomycin usage (DDDs/1000 patient bed-days) before and after the introduction of IDEA3S are shown in Box 3.
Disturbingly, ceftriaxone/cefotaxime use increased after the initial introduction of IDEA3S, until it was realised that IDEA3S contained an incorrect dosage recommendation for community-acquired pneumonia (2 g daily instead of 1 g daily10). Once this error was corrected, ceftriaxone/cefotaxime use decreased promptly to pre-IDEA3S rates, where it has remained (Box 3). Vancomycin use remained stable throughout the study period (Box 3), despite ongoing problems with nosocomial methicillin-resistant Staphylococcus aureus infection at Austin Health (unpublished data).
Among the 130 patients reviewed in the 9-month audit of concordance between the IDEA3S recommendations for treating community-acquired pneumonia and actual anti-biotic use, “exact concordance” and “concordance” were noted in 66 and 33 patients, respectively (76% of total); “non-concordance” was observed in 31 patients.
We found IDEA3S to be a clinically practical, readily accepted means of maintaining appropriate antibiotic prescribing levels at our institution. Unlike other electronic decision-support and approval systems that have been used to help control known high rates of inappropriate antibiotic use,11 IDEA3S was introduced into a clinical environment in which tight antibiotic controls were already established. In this setting, the level of key antibiotic use before introduction of IDEA3S was maintained after its introduction. At the same time, the use of IDEA3S significantly reduced the burden associated with the wholly phone-based approval system.
We were initially disappointed with the 48% rate at which IDEA3S approvals substituted for phone-based approvals. However, review of phone-based approval calls suggested that a large proportion of those initially considered to be solely for antibiotic approval in fact included detailed clinical discussion and required general infectious disease advice in addition to simple drug approval. Such discussions and advice are an important role for any infectious diseases department, and it was never intended that IDEA3S should replace this service. This observation has important implications for hospitals considering the introduction of a system such as IDEA3S: although electronic approval systems may provide some decision support, they should not be expected to replace the need for specialist consultation.
We found the ability of IDEA3S to record details about prescribers, patients and the antimicrobials and indications requested a major advantage. Firstly, it meant that clinicians prescribing inappropriately could be readily identified and targeted for educational activities. On some occasions, feedback from prescribers suggested that problem prescribing was not at the level of hospital medical officers or registrars, but rather at the level of senior consultants, some of whom had inappropriate or unusual views about appropriate antibiotic selection. In such cases, our educational efforts were altered accordingly (eg, we had discussions with some individual consultants). Secondly, we could easily identify prescribers who “gamed” the system by untruthfully stating that their patient had a condition qualifying for antibiotic approval. Since IDEA3S can be linked to the Medical Records Department database, patient audits could be readily undertaken (using the ICD-10 disease coding identification system) to identify whether the patient had the stated condition. Hospital-unit-based or prescriber-based audits for prescribing accuracy can be automated to provide regular feedback and can potentially be used as a quality assessment tool. Thirdly, IDEA3S allowed ready identification of good prescribers. At Austin Health, these prescribers were identified/congratulated at medical grand rounds. Finally, for community-acquired pneumonia, the ability of IDEA3S to calculate Pneumonia Severity Index (PSI) scores and store them allowed us to audit the accuracy of these entries and identify any systematic clinician errors or misunderstandings when using this tool. Before IDEA3S, such audits were not feasible, whereas our computer-based audit could be completed by one pharmacist in a few hours.
Notably, successful introduction of a system such as IDEA3S requires commitment and careful consideration of local institutional features. Staff turnover and rostering means that users constantly change, and regular education is essential to maintain use of any decision-support tool.
The increasing complexity of in-hospital healthcare, associated with reduced in-hospital length of stay, emergence of significant antibiotic-resistant pathogens and increased drug costs, all reinforce the need to ensure appropriate antibiotic prescribing. IDEA3S not only helped to achieve this goal, but is well placed to link to future electronic prescribing systems. We have now rewritten IDEA3S into a Windows-based format that will be suitable for use in most Australian hospitals and obtainable at no cost from our website (www.debug.net.au).
1: Antimicrobial approval requests (“hits”) on IDEA3S at Austin Health, March 2002–August 2003*
IDEA3S = infectious diseases electronic antibiotic advice and approval system.
* Six other IDEA3S-listed agents (aciclovir, ceftazidime, famciclovir, ticarcillin/clavulanic acid, piperacillin, piperacillin/tazobactam) each had less than 25 hits during this period.
2: Approvals for two antibiotics (cefotaxime/ceftriaxone and vancomycin) dispensed by the Pharmacy Department before and after the introduction of IDEA3S
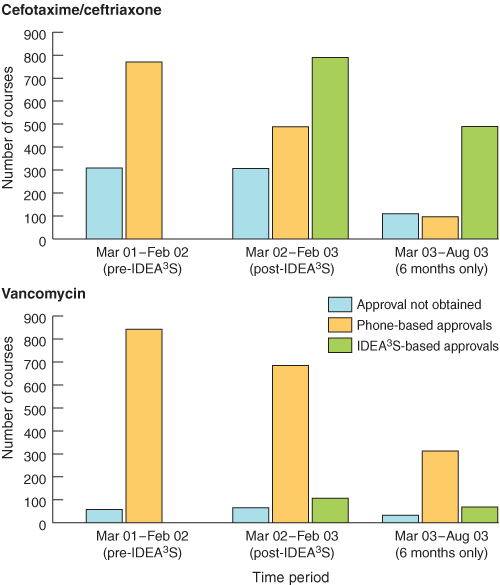
IDEA3S = infectious diseases electronic antibiotic advice and approval system.
3: Impact of IDEA3S (“hits”/month) on the use of ceftriaxone/cefotaxime and vancomycin (expressed as defined daily doses/1000 patient bed-days) at Austin Health, March 2002–August 2003*†
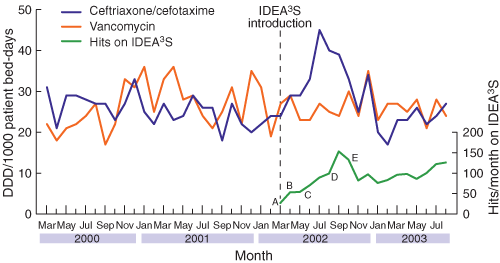
DDD = defined daily doses. IDEA3S = infectious diseases electronic antibiotic advice and approval system.
* Ceftriaxone/cefotaxime use increased after the initial introduction of IDEA3S, until it was realised that IDEA3S contained an incorrect dosage recommendation for community-acquired pneumonia (2 g daily instead of 1 g daily).
† An educational program was introduced at various stages for specific staff/departments: interns and surgeons (A), medical registrars (B), interns (C), Emergency Department (D); medical grand rounds (E).
4: Requirements for successful introduction of a clinical electronic advice and approval system
Coordinated team approach involving input from clinicians; physicians with expertise in appropriate antibiotic prescribing; departments of infectious disease, pharmacy and information technology; health information services and hospital administration.
Acceptance by medical staff and other key stakeholders that evidence-based recommendations/controls on antibiotic prescribing are important.
A coordinated education program conducted by infectious diseases staff (or designated clinical leader[s]), initially targeting key prescribers (interns, hospital medical officers, registrars, senior consultants). Educational efforts need to be repeated regularly to ensure all staff (regardless of their roster) are educated about the electronic advice and approval system.
Appropriate time allocation for clinicians and pharmacy staff to conduct education programs (estimated to require 0.1–0.2 and 0.5 full-time-equivalent staff, respectively).
Appropriate computer access for clinician prescribers.
A user-friendly computer interface.
A hospital-based intranet (preferred, but not mandatory). Intranet access improves potential linkages with the medical records department to improve auditing capabilities.
Pharmacy department adherence to the rule of not supplying restricted antibiotics to prescribers unless an approval number has been obtained. Removal of restricted antibiotics from wards.
Regular pharmacy department audits of the use of key antibiotics to ensure appropriate antibiotic usage and clinician compliance with the advice and approval system.
Feedback of audit data to prescribers to improve compliance with evidence-based guidelines and focus targeted education efforts to improve appropriate prescribing.
- M Lindsay Grayson1
- Sharmila Melvani2
- Sue W Kirsa3
- Stephen Cheung4
- M Kent Garrett5
- Anthony M Korman6
- William A Thomson7
- 1 Austin Health, Heidelberg, VIC.
- 2 Monash Medical Centre, Clayton, VIC.
- 3 Victorian Drug Usage Advisory Committee, Carlton South, VIC.
We wish to acknowledge the assistance of infectious diseases registrars who collected phone-based antibiotic approvals information before and after the introduction of IDEA3S and the many members of the Infectious Diseases, Pharmacy, Respiratory Medicine, Surgery and Emergency departments at Austin Health who provided valuable suggestions for both developing and improving IDEA3S. Finally, we would like to thank clinicians for using IDEA3S. Our survey was funded by a Quality Improvement Grant from the Department of Human Services, Victoria.
None identified.
- 1. McManus P, Hammond ML, Whicker SD, et al. Antibiotic use in the Australian community, 1990–1995. Med J Aust 1997; 167: 124-127. <MJA full text>
- 2. World Health Organization. WHO global strategy for containment of antimicrobial resistance. Geneva: WHO, 2001.
- 3. World Health Organization report on infectious diseases 2000: overcoming antimicrobial resistance. Chapter 3: factors contributing to resistance. WHO, 2000. Available at: www.who.int/infectious-disease-report/2000 (accessed Mar 2004).
- 4. Robertson MB, Dartnell JG, Korman TM, on behalf of the Victorian Drug Usage Evaluation Group. Vancomycin and teicoplanin use in Victorian hospitals. Med J Aust 1999; 171: 127-131. <MJA full text>
- 5. Robertson MB, Korman TM, Dartnell JG, et al, on behalf of the Victorian Drug Usage Evaluation Group. Ceftriaxone and cefotaxime use in Victorian hospitals. Med J Aust 2002; 176: 524-529. <MJA full text>
- 6. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336: 243-250.
- 7. Johnson PDR, Irving LB, Turnidge JD. Community-acquired pneumonia. Med J Aust 2002; 176: 341-347. <MJA full text>
- 8. Anthonisen NR, Manfreda J, Warren CP, et al. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106: 196-204.
- 9. World Health Organization. ATC index with DDDs. Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology, 2003. Available at: www.whocc.no/atcddd (accessed Mar 2004).
- 10. Therapeutic guidelines: antibiotic. 12th ed. Melbourne: Therapeutic Guidelines Limited, 2003.
- 11. Richards MJ, Robertson MB, Dartnell JG, et al. Impact of a web-based antimicrobial approval system on broad-spectrum cephalosporin use at a teaching hospital. Med J Aust 2003; 178: 386-390. <MJA full text>





Abstract
The impact of a computer-based infectious diseases electronic antibiotic advice and approval system (“IDEA3S”) was assessed as an alternative to a labour-intensive, phone-based approval system.
IDEA3S-based approvals replaced 48% of all approvals for the most frequently requested antimicrobial agents (ceftriaxone/cefotaxime, vancomycin) and were associated with stable overall rates of antimicrobial use.
Antibiotic prescribing for community-acquired pneumonia was 76% concordant with IDEA3S recommendations, and clinical acceptance of IDEA3S was excellent.
Successful implementation required a coordinated, evidence-based approach between clinicians, pharmacists and hospital administration, together with ongoing staff education and feedback of results.
IDEA3S is a useful new adjunct to routine clinician consultation to support appropriate antibiotic prescribing for a number of common indications in hospitals.