Few infections generate as much controversy as community-acquired pneumonia. Reasons for this include the range of possible pathogens, difficulty in determining which pathogen to target when choosing an antibiotic, the variety of available antibiotics and increasing antibiotic resistance. In this article, we have tried to balance the needs of the individual patient with the need to control healthcare costs and antibiotic resistance. Our recommendations are restricted to the management of adults.
Community-acquired pneumonia (CAP) is commonly defined as an acute infection of the lower respiratory tract occurring in a patient who has not resided in a hospital or healthcare facility in the previous 14 days.1 Current approaches to the empirical management of CAP emphasise the type of patient ("community" or "hospital"), rather than the type of symptoms ("typical" or "atypical").
We lack detailed information on the incidence of CAP in Australia, but in the United States CAP requiring hospital admission occurs in about 258 per 100 000 population per year, rising to 962 per 100 000 among those aged 65 years or over.1 Mortality rates in recent years appear to have increased. Mortality averages 14%, but is less than 1% for those not requiring admission to hospital.1
Although inhalation and micro-aspiration constantly deliver potential pathogens, the respiratory tract below the larynx is normally sterile. Sterility is maintained by host defence systems, which include innate and acquired immunity and the mucociliary transport system. Factors that perturb these systems or predispose to aspiration increase the risk of pneumonia.
In community studies in Finland, the rate of CAP increased for each year of age over 50 years; other risk factors were alcoholism, asthma, immunosuppression, and institutionalisation.1 In the United States, studies of risk factors for infection with Streptococcus pneumoniae have implicated dementia, seizure disorders, smoking, heart failure, stroke and chronic obstructive pulmonary disease.2 In Australia, Indigenous people have an increased risk of admission to hospital with CAP3,4 and of pneumococcal pneumonia5 (Box 1). Studies in Victoria have shown that pneumococcal pneumonia is common in active elderly people, not only in the sick and infirm.6
Many pathogens can cause CAP. A South Australian study of 106 adults admitted to hospital with CAP in 1987–1988 found that the most common cause was S. pneumoniae ("pneumococcus") (42%), followed by respiratory viruses (18%), Haemophilus influenzae (9%), Mycoplasma pneumoniae and enteric gram-negative bacteria (8% each), Chlamydia psittaci (5%), Staphylococcus aureus, Legionella spp. and Mycobacterium tuberculosis (3% each).7 More recent overseas studies have shown that S. pneumoniae is still the most common pathogen overall, followed possibly by M. pneumoniae and Chlamydia pneumoniae.1,2 In ambulatory care, the proportion of patients with pathogens such as M. pneumoniae and C. pneumoniae that do not respond to penicillin, amoxycillin or cephalosporins may approach 50%.8
Race, geographic location, lifestyle and country of origin influence the expected aetiology of CAP. For example, pneumococcal pneumonia occurs at high rates in Indigenous Australians, while Burkholderia pseudomallei (melioidosis) and Acinetobacter baumanii are important causes of CAP in people in tropical Australia,9,10 as is tuberculosis in people born overseas. HIV infection should be considered in patients with recurrent pneumococcal pneumonia. Pneumocystis carinii infection may be the cause of an unusually prolonged dry cough in a patient with HIV risk factors.
Aspiration pneumonia is an important variant of community-acquired pneumonia that occurs particularly in elderly people and those with conditions such as bulbar weakness, laryngectomy or stroke. Pulmonary segments that are lowermost at the moment of aspiration are involved. The most common causative organisms identified in recent studies were S. aureus, H. influenzae and gram-negative aerobes. Contrary to standard teaching, no anaerobes were found.11,12
CAP should be considered when a patient presents with two or more of the following symptoms:
fever;
rigors;
new-onset cough;
change in sputum colour if there is a chronic cough;
chest discomfort; or
dyspnoea.
However, many patients who satisfy these criteria do not have pneumonia, and failure to distinguish pneumonia from acute bronchitis is an important reason for overuse of antibiotics.1,2 Furthermore, CAP can present with fever without localising features, and some patients may have no fever (eg, elderly patients may present only with a sudden change in functional status).
Thus, if pneumonia is being considered, a chest x-ray is needed. No set of decision rules is as yet superior to clinical judgement when deciding whom to x-ray.13 Physical signs of consolidation are suggestive but are often not found at presentation. Nevertheless, some clinical signs, such as confusion, should be specifically noted because of their prognostic value14,15 (see Risk stratification).
This is the cardinal investigation. In the appropriate setting, a new area of consolidation on chest x-ray makes the diagnosis, but x-ray is a poor guide to the likely pathogen. Other causes of a new lung infiltrate on chest x-ray include atelectasis, non-infective pneumonitis, haemorrhage and cardiac failure. Occasionally, the chest x-ray initially appears normal (eg, in the first few hours of S. pneumoniae pneumonia and early in HIV-related P. carinii pneumonia) (Box 2).
There is debate about the value of sputum samples in diagnosis of CAP. Oral flora rather than the offending pathogen may dominate a sputum Gram stain and culture. Nevertheless, we believe that an attempt should be made to obtain a sputum sample before beginning antibiotic therapy, as this is sometimes the best opportunity to identify pathogens that need special treatment. Microscopy and culture for M. tuberculosis should be requested if the patient was born overseas.
All patients with CAP being assessed in emergency departments or admitted to hospital should have oximetry, measurement of serum electrolytes and urea levels, and a full blood count to assist in assessing severity. Blood gas measurement is also recommended, as it provides prognostic information (pH and Pao2) and may identify patients with ventilatory failure or chronic hypercapnia (Paco2). If the patient has known or suspected diabetes mellitus, measurement of blood glucose also assists in assessing severity.
Blood cultures are the most specific diagnostic test for the causative organism, but are positive in only around 10% of patients admitted to hospital with CAP.1 The more severe the pneumonia, the more likely blood cultures are to be positive.16 We recommend that blood be cultured from all patients, except those well enough to be managed at home with oral antibiotics.
The Legionella urinary antigen test is rapid, reliable and has a high degree of sensitivity and specificity.17 It should be performed in all patients with CAP, except perhaps those with low enough risk to be managed at home with empirical oral therapy (see Risk stratification). However, the test detects only Legionella pneumophila serogroup 1, which accounts for only half of all cases of Legionella pneumonia.
Viral immunofluorescence testing of a nasopharyngeal aspirate is rapid and useful if it detects influenza or respiratory syncytial virus. Virus detection does not preclude a secondary bacterial invader.
Serological diagnosis requires acute and convalescent serum samples and is therefore not useful in acute management of CAP. Some laboratories offer acute serodiagnosis for M. pneumoniae, but these tests may lack specificity.18
Even after extensive investigations, the microbial cause of CAP is revealed in only about half of all patients.1,2 New diagnostic tests are under development. The most promising are rapid screens that can be performed on throat swabs, using polymerase chain reaction.
CAP is common, and many patients will recover with a simple oral antibiotic regimen, or even without antibiotics. However, a small proportion are at significant risk of death. Questions to be considered after radiological confirmation of CAP are:
What is the severity?
Where should the patient be managed?
Which antibiotics should be used?
Risk-stratification systems can help answer these questions. One approach is to refer to a list of mortality risk factors (Box 3). A New Zealand study found that patients with CAP who had at least two key features on admission (diastolic blood pressure ≤ 60 mmHg, respiratory rate ≥ 30 per minute, serum urea level > 7 mmol/L, or confusion) were 36 times more likely to die than those without these features.15
In the United States, a prospectively validated severity prediction score is increasingly used — the Pneumonia Severity Index (PSI).19,20 The method of scoring this index is shown in Box 4, and risk of death in different PSI risk classes in Box 5. The rule was derived in patients aged over 18 years who were HIV-antibody negative and had not been in hospital during the previous seven days, although they included nursing home residents. Strictly, the PSI score identifies predictors of mortality and was not originally designed to triage patients or guide prescribing. However, high PSI scores correlate with admission to hospital and an intensive care unit, and there is limited evidence that the score correctly identifies patients who can be safely managed in the community with oral antibiotics.20
A suggested protocol for determining patient risk and management using the PSI score is shown in Box 6. We recommend that all but the lowest-risk patients (PSI risk class I) be further assessed. Whenever practicable, this assessment should be in an emergency department with rapid access to laboratory results. To apply the PSI algorithm, blood pH must be estimated; while pulse oximetry measurement of O2 saturation can substitute for po2, until recently there has been no alternative to arterial blood gas measurement to assess pH. A recent Australian study showed that pH obtained by rapid analysis of a venous blood sample is a good approximation of arterial blood pH.21 Therefore, if arterial blood gas cannot be measured, O2 saturation plus venous blood pH could be substituted.
Risk-stratification systems, such as the PSI score, should not replace good clinical judgement. For example, a homeless low-risk patient should not be sent "home" on oral antibiotics, and a patient who is vomiting should not be treated with oral therapy. In addition, the original description of the PSI score contained the important caveat that all patients with hypoxia in room air (O2 saturation < 90% or po2 < 60 mmHg) or unusual comorbidities not specifically scored (eg, severe neuromuscular disease) should be admitted to hospital, regardless of PSI score.19
In Australia, some organisms that cause CAP are increasingly resistant to antibiotics. However, laboratory resistance does not automatically correlate with treatment failure. For example, although about 20% of clinical isolates of S. pneumoniae now have reduced susceptibility to penicillin,22 most of this resistance is "intermediate", meaning that CAP is likely to respond to oral amoxycillin or parenteral benzylpenicillin. Clinical failure of penicillins in respiratory infection caused by S. pneumoniae is unlikely unless the penicillin minimum inhibitory concentration exceeds 4 mg/L (high-level resistance).23 Such strains are still rare in Australia. In contrast, treatment has failed in cases of meningitis caused by S. pneumoniae with intermediate resistance,24 because of the additional problem of drug penetration to the cerebrospinal fluid. Third-generation cephalosporins may also fail against these strains. S. pneumoniae may also be resistant to trimethoprim–sulfamethoxazole (42% of clinical isolates), tetracyclines (15%) and erythromycin (11%).25
Resistance to amoxycillin is steadily increasing in H. influenzae and is currently about 25%. Resistance of Mycoplasma, Chlamydia and Legionella species to their drugs of choice is rare.
For patients at low risk (risk class I and many patients in classes II and III, corresponding to PSI score < 90), management with oral antibiotic therapy in the community is probably appropriate, provided they are not hypoxic and their social circumstances are suitable. Regular review is essential. We recommend a combination of amoxycillin and either roxithromycin or doxycycline (the latter should be avoided in pregnancy).
Amoxycillin is aimed at S. pneumoniae, as this is still the single most likely pathogen, and is preferable to penicillin as absorption and dosing frequency are more favourable. Doxycycline and roxithromycin are usually effective against other potential pathogens not covered by amoxycillin, which are common in ambulatory patients. Resistance of S. pneumoniae to these agents is more likely to be clinically significant than resistance to penicillin or amoxycillin. We believe that pneumococcal resistance to roxithromycin and doxycycline is now too common in Australia to recommend use of one of these agents alone for CAP. In this respect, our recommendations differ from those of the 11th version of Therapeutic guidelines: antibiotic.26
In patients with penicillin allergy, oral cephalexin or cefaclor should probably not be used, as their coverage of S. pneumoniae with reduced penicillin susceptibility is suboptimal. Sole reliance on a macrolide or tetracycline is also not recommended for the reasons above. An option for these patients is a combination of either oral cefuroxime axetil or outpatient intravenous ceftriaxone (which can be given once daily) with oral roxithromycin or doxycycline. Another option is single-agent therapy with one of the new fluoroquinolones — moxifloxacin or gatifloxacin — as these agents are effective against all common pathogens. However, they are not yet available on the Pharmaceutical Benefits Scheme, are expensive compared with standard oral therapy, and their overuse could generate resistance to valuable reserve agents, such as ciprofloxacin.
Patients at higher risk (PSI risk class IV and some patients in other classes) require intravenous therapy. Intravenous benzylpenicillin plus oral roxithromycin or doxycycline still provide excellent cover for almost all pathogens, except S. aureus and gram-negative organisms (case report, Box 7). Flucloxacillin or dicloxacillin should be added if staphylococcal pneumonia is suspected (ie, recent influenza, sputum Gram stain shows gram-positive cocci resembling staphylococci, or blood or sputum cultures yield S. aureus). Similarly, gram-negative rods in the sputum or blood should prompt immediate addition of an aminoglycoside or extended-spectrum cephalosporin.
For patients at highest risk of death (PSI risk class V), early broad-spectrum parenteral therapy is essential. Failure to include antibiotics effective against the pathogen in the initial regimen worsens prognosis.1
Intravenous erythromycin plus ceftriaxone or cefotaxime has been recommended for severe CAP by Therapeutic guidelines: antibiotic for several years. In contrast, the 11th (2000) version recommends intravenous erythromycin plus penicillin and gentamicin, with the previous regimen reserved for patients with penicillin allergy.24 A recent non-randomised comparison in Australia suggested that the two regimens may be equivalent,27 but a properly powered, randomised study is required to settle the issue.28 Until better evidence is available, we continue to recommend intravenous erythromycin plus either ceftriaxone or cefotaxime in these very unwell patients. It is also necessary to consider carefully the possibility of specific pathogens which may require additional therapy (eg, S. aureus, Pseudomonas spp., other gram-negative organisms and P. carinii).
Patients in tropical Australia, particularly those with more severe pneumonia, may be infected with B. pseudomallei (melioidosis) or A. baumannii and thus may require different initial empirical therapy. Patients with CAP in risk classes III or IV who also have risk factors for these infections (eg, diabetes, chronic airways disease, high alcohol intake or renal disease) should receive initial therapy with regimens that include intravenous gentamicin plus ceftriaxone (2 g for adults). All patients in risk class V should receive regimens that include intravenous gentamicin plus meropenem, if available. The regimen needs to be further refined if one of these pathogens is identified.24 [This paragraph corrected on 5 August 2002- click here for previous wording]
For patients with hypoxaemia, continuous oxygen therapy should be provided with the aim of maintaining O2 saturation over 95% (or 90% in those with chronic hypercapnia). Patients with asthma or chronic obstructive pulmonary disease require optimisation of their bronchodilator therapy. Adequate hydration is also important, but care should be taken in older patients to avoid fluid overload, which may worsen gas exchange. Specific therapy for cardiac failure may be required. Occasionally, patients with severe pneumonia develop acute renal failure, which may require temporary dialysis. Changes in renal function should be kept in mind when selecting antibiotics and antibiotic doses.
Patients with chest pain require pain control to facilitate coughing and clearance of secretions, but routine chest physiotherapy is probably not useful unless secretions are copious. Adequate humidification of inspired air and suctioning of the large airways in patients with reduced consciousness or poor cough may also be useful.
Patients need to be monitored clinically to ensure that their condition improves on treatment. Daily review for the first few days is recommended. Improvement on chest x-ray is often slow and should not be used to monitor initial response to treatment. Improved sense of well-being, reduced temperature and reduced respiratory rate are expected in most patients in 24 to 72 hours, but may take longer if pneumonia is severe.14 Failure to improve should prompt review of the case. Antibiotic failure itself is not usually the reason.
If the patient's condition does not improve, the following should be considered:
Is the diagnosis correct? (Results of diagnostic tests should be rechecked.)
Is the patient taking the antibiotics?
Would hospital admission and intravenous therapy now be appropriate?
Is there a complication (eg, effusion or empyema)?
Is there obstruction (eg, bronchial carcinoma or a foreign body)?
Is the pathogen S. aureus, Pseudomonas spp. or other gram-negative rod, which may not respond to standard empirical regimens?
Is it tuberculosis?
Could the patient have HIV infection?
Should the patient be referred to a specialist (eg, for diagnostic bronchoscopy)?
Annual influenza vaccination and five-yearly pneumococcal vaccination are recommended for people with risk factors and all those aged over 65 years.29 For Indigenous people, who have much higher rates of CAP than the non-Indigenous population, regular influenza and pneumococcal vaccination is recommended from the age of 50.29
Legionnaire's disease, tuberculosis and psittacosis are notifiable diseases. Suspected cases should be reported immediately to local public health authorities so that public health measures can be taken.
Evidence-based recommendations
-
Certain patient, clinical and laboratory features at presentation are independently associated with risk of death from community-acquired pneumonia14 ,19 (E3 2 ).
-
These features can be used to generate a pneumonia severity index which correlates with risk of death, need for hospital admission, length of hospital stay and need for intensive care19 (E3 2 ).
-
The pneumonia severity index can be used with caution to guide decisions about where and how to manage patients20 (E3 3 ).
1: Factors that increase risk of community-acquired pneumonia2-5
Age over 50 years Alcoholism Asthma Chronic obstructive pulmonary disease Dementia Heart failure |
Immunosuppression Indigenous background Institutionalisation Seizure disorders Smoking Stroke |
2: Chest x-ray in Streptococcus pneumoniae pneumonia
Chest x-ray is the cardinal investigation in community-acquired pneumonia, but may occasionally be misleading.
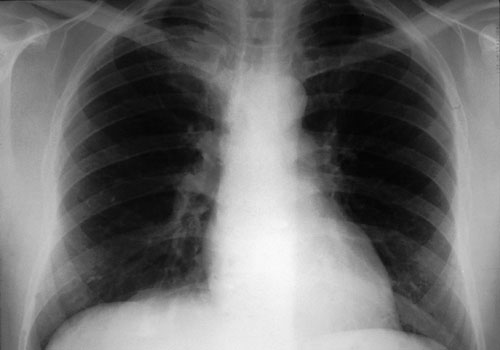
At presentation: A 47-year-old smoker presented after just a few hours of rigors and productive cough. Despite clinical signs of right upper zone consolidation, chest x-ray showed only minor abnormalities. Empirical therapy for community-acquired pneumonia was begun.
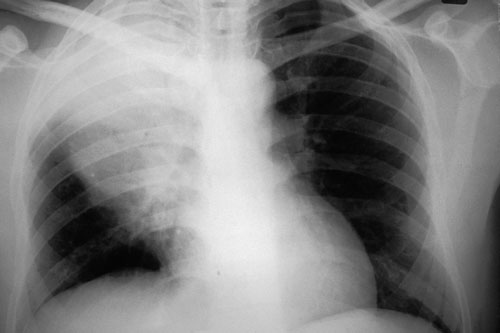
12 hours later: Chest x-ray showed consolidation in the right upper lobe consistent with the earlier clinical signs. S. pneumoniae was isolated from blood cultures. The patient recovered fully. (X-rays courtesy of Dr Bryan Speed, Fairfield Hospital Historical Collection, Melbourne, VIC.)
3: Factors that predict increased risk of death from community-acquired pneumonia14
Factor |
Odds ratio for death |
||||||||||
Identifiable at initial assessment |
|||||||||||
Hypothermia (temperature ≤ 37°C) |
5.0 |
||||||||||
Hypotension (systolic blood pressure < 100 mmHg) |
4.8 |
||||||||||
Existing neurological disease |
4.6 |
||||||||||
More than one lobe involved on chest x-ray |
3.1 |
||||||||||
Tachypnoea (respiratory rate ≥ 20 per min) |
2.9 |
||||||||||
Existing neoplastic disease |
2.8 |
||||||||||
Leukopenia (white cell count ≤ 10 x 109/L) |
2.5 |
||||||||||
Confusion |
2.3 |
||||||||||
Diabetes mellitus |
1.3 |
||||||||||
Male sex |
1.3 |
||||||||||
Other factors |
|||||||||||
Bacteraemia |
|||||||||||
Specific causative organisms: |
|||||||||||
Pseudomonas aeruginosa |
|||||||||||
Other gram-negative rods (eg, Escherichia coli, Klebsiella spp.) |
|||||||||||
Staphylococcus aureus |
|||||||||||
Legionella pneumophila |
|||||||||||
4: Patient classification using Pneumonia Severity Index (PSI)19
PSI risk class I (lowest risk). Patient has none of the following:
Age > 50 years;
History of neoplastic disease, congestive cardiac failure, cerebrovascular, renal or liver disease; or
Clinical signs — altered mental state, pulse rate ≥ 125 per minute, respiratory rate ≥ 30 per minute, systolic blood pressure < 90 mmHg, or temperature < 35°C or ≥ 40°C.
PSI risk classes II–V. Patients with any of the above characteristics are classified according to their PSI score, calculated according to the table below.
Calculation of PSI risk score |
|||||||||||
Factor |
PSI score |
||||||||||
Patient age |
Age in years (male) or age – 10 (female) |
||||||||||
Nursing home resident |
+10 |
||||||||||
Coexisting illnesses |
|||||||||||
Neoplastic disease |
+30 |
||||||||||
Liver disease |
+20 |
||||||||||
Congestive cardiac failure |
+10 |
||||||||||
Cerebrovascular disease |
+10 |
||||||||||
Renal disease |
+10 |
||||||||||
Signs on examination |
|||||||||||
Altered mental state |
+20 |
||||||||||
Respiratory rate ≥ 30 per minute |
+20 |
||||||||||
Systolic blood pressure < 90 mmHg |
+20 |
||||||||||
Temperature ≤ 35°C or ≥ 40°C |
+15 |
||||||||||
Pulse rate ≥ 125 bpm |
+10 |
||||||||||
Results of investigations |
|||||||||||
Arterial pH < 7.35 |
+30 |
||||||||||
Serum urea level ≥ 11 mmol/L |
+20 |
||||||||||
Serum sodium level < 130 mmol/L |
+10 |
||||||||||
Serum glucose level ≥ 14 mmol/L |
+10 |
||||||||||
Haematocrit < 30% |
+10 |
||||||||||
Po2 < 60 mmHg or O2 saturation < 90% |
+10 |
||||||||||
Pleural effusion |
+10 |
||||||||||
5: Mortality within 30 days according to PSI risk class19
Risk class |
Score |
Mortality |
|||||||||||||
I |
Score not calculated |
0.1% |
|||||||||||||
II |
≤ 70 |
0.6% |
|||||||||||||
III |
71–90 |
0.9% |
|||||||||||||
IV |
91–130 |
9.3% |
|||||||||||||
V |
>130 |
27.0% |
|||||||||||||
PSI = Pneumonia Severity Index. |
|||||||||||||||
6: Diagnosis and management of community-acquired pneumonia in adults*
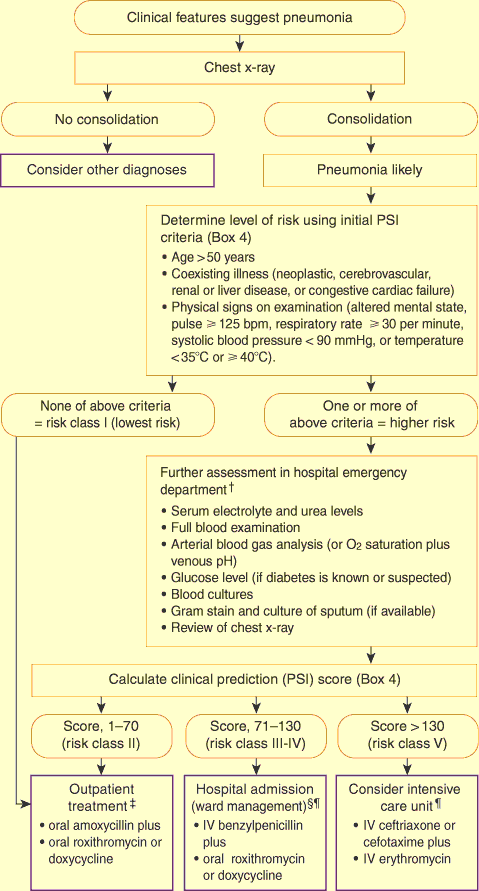
PSI = Pneumonia Severity Index. IV = intravenous.
* Adapted from Fine et al.19
† Assessment in the community may be appropriate if access to an emergency department is limited by time or distance.
‡ With the exception that patients with hypoxia in room air (O2 saturation < 90% or po2 < 60 mmHg) should be admitted to hospital whatever their PSI score.
§ If blood pH has been measured, and score is < 90, then patient may be considered for management as an outpatient with oral therapy.
[This footnote corrected on 5 August 2002 - click here for previous wording]
7: Case report — management of community-acquired pneumonia
Presentation: A 66-year-old man, accompanied by his wife, presented to his general practitioner with three days of fever, cough and increasing breathlessness. He had a past history of smoking, chronic airways disease and type 2 diabetes. He was born in Australia, had no recent history of travel overseas or to northern Australia, or of influenza-like illness. Examination: The patient was not able to give a coherent history. His temperature was 38.0°C; blood pressure, 140/70; pulse rate, 120 bpm; and respiratory rate, 30 per minute. He had widespread coarse lung crackles and wheeze but no focal signs. Investigations: Clinical assessment suggested pneumonia. An urgent chest X-ray showed consolidation in the left lingular and right lower lobe. As the clinical picture suggested severe pneumonia, the patient was referred immediately to the nearest hospital emergency department for further assessment. Risk assessment and management: The patient had at least two characteristics preventing classification as low risk (age > 50 years and confusion). Sputum Gram stain and culture, blood cultures, measurement of serum urea and electrolyte levels, liver function tests, full blood examination and Legionella urinary antigen tests were ordered. Based on results, PSI score was calculated as 126, or risk class IV (see table at right). High flow oxygen was begun to keep O2 saturation > 95%, and fluid and insulin were given for diabetes. Intravenous penicillin (1.8 g, 4-hourly) plus oral doxycycline (200 mg oral loading dose, then 100 mg twice daily) were prescribed. Day 2: Legionella urinary antigen test gave a positive result, and the local public health unit was notified. As the patient was still unwell, oral doxycycline was replaced with intravenous erythromycin (1 g, 6-hourly), and penicillin was continued. Day 3: The patient's condition began to improve, but fever persisted. Day 5: The patient was afebrile for the first time. Day 7: His condition had improved further, and therapy was changed to oral doxycycline because of phlebitis. Antibiotic treatment was continued until Day 14. |
Calculation of PSI score* |
||||||||||
Factor |
Result |
PSI score |
|||||||||
Patient age |
66 years |
+ 66 |
|||||||||
Specified coexisting illness |
No |
0 |
|||||||||
Signs on examination |
|||||||||||
Confusion |
Yes |
+ 20 |
|||||||||
Respiratory rate |
28 per min |
0 |
|||||||||
Systolic blood pressure |
140 mmHg |
0 |
|||||||||
Temperature |
38°C |
0 |
|||||||||
Pulse rate |
120 bpm |
0 |
|||||||||
Results of investigations |
|||||||||||
Serum urea level |
17 mmol/L |
+ 20 |
|||||||||
Serum sodium level |
136 mmol/L |
0 |
|||||||||
Serum glucose level |
19.6 mmol/L |
+ 10 |
|||||||||
Haematocrit |
40% |
0 |
|||||||||
O2 saturation |
86% |
+ 10 |
|||||||||
pH |
7.36 |
0 |
|||||||||
Pleural effusion |
No |
0 |
|||||||||
Total |
126 | ||||||||||
* If results of blood tests cannot be obtained rapidly (eg, in remote areas), risk can be determined without the PSI score (see Box 3). In this case, presence of diabetes, respiratory rate > 20 per minute, confusion and multilobar disease on x-ray would have suggested that the patient was at significantly increased risk of death. |
|||||||||||
- Paul D R Johnson1
- Lou B Irving2
- John D Turnidge3
- 1 Austin and Repatriation Medical Centre, Melbourne, VIC.
- 2 Department of Microbiology and Infectious Diseases, Women's and Children's Hospital, Adelaide, SA.
- 1. Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Clin Infect Dis 2000; 31: 347-382.
- 2. Mandell LA, Marrie TJ, Grossman RF, et al, and the Canadian Community-Acquired Pneumonia Working Group. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clin Infect Dis 2000; 31: 383-421.
- 3. Thompson JE. Community acquired pneumonia in north eastern Australia — a hospital based study of aboriginal and non-aboriginal patients. Aust N Z J Med 1997; 27: 59-61.
- 4. Williams P, Gracey M, Smith P. Hospitalization of aboriginal and non-aboriginal patients for respiratory tract diseases in Western Australia, 1988–1993. Int J Epidemiol 1997; 26: 797-805.
- 5. Trotman J, Hughes B, Mollison L. Invasive pneumococcal disease in central Australia. Clin Infect Dis 1995; 20: 1553-1556.
- 6. Padiglione AA, Willis J, Bailey M, Fairley CK. Characteristics of patients with community-acquired pneumococcal pneumonia. Med J Aust 1999; 170: 165-167.
- 7. Lim I, Shaw DR, Stanley DP, et al. A prospective hospital study of the aetiology of community-acquired pneumonia. Med J Aust 1989; 151: 87-91.
- 8. Marrie TJ, Peeling RW, Fine MJ, et al. Ambulatory patients with community-acquired pneumonia: the frequency of atypical agents and clinical course. Am J Med 1996; 101: 508-515.
- 9. Currie BJ, Fisher DA, Howard DM, et al. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis 2000; 31: 981-986.
- 10. Anstey NM, Currie BJ, Withnall KM. Community-acquired Acinetobacter pneumonia in the Northern Territory of Australia. Clin Infect Dis 1992; 14: 83-91.
- 11. Marik PE. Primary care: aspiration pneumonitis and aspiration pneumonia. N Engl J Med 2001; 344: 665-671.
- 12. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest 1999; 115: 178-183.
- 13. Emerman CL, Dawson N, Speroff T, et al. Comparison of physician judgement and decision aids for ordering chest radiographs for pneumonia in outpatients. Ann Emerg Med 1991; 20: 1215-1219.
- 14. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 1996; 275: 134-141.
- 15. Neill AM, Martin IR, Weir R, et al. Community acquired pneumonia: aetiology and usefulness of severity criteria on admission. Thorax 1996; 51: 1010-1016.
- 16. Waterer GW, Wunderink RG. The influence of the severity of community-acquired pneumonia on the usefulness of blood cultures. Respir Med 2001; 95: 78-82.
- 17. Stout JE, Yu VL. Legionellosis. N Engl J Med 1997; 337: 682-687.
- 18. Jacobs E. Serological diagnosis of Mycoplasma pneumoniae infections: a critical review of current procedures. Clin Infect Dis 1993; 17 Suppl 1: S79-S82.
- 19. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med 1997; 336: 243-250.
- 20. Atlas SJ, Benzer TI, Borowsky LH, et al. Safely increasing the proportion of patients with community-acquired pneumonia treated as outpatients. Arch Intern Med 1998; 158: 1350-1356.
- 21. Kelly AM, McAlpine R, Kyle E. Venous pH can safely replace arterial pH in the initial evaluation of patients in the emergency department. Emerg Med J 2001; 18: 340-342.
- 22. Collignon PJ, Turnidge JD. Antibiotic resistance in Streptococcus pneumoniae. Med J Aust 2000; 173 Suppl: S58-S64.
- 23. Heffelfinger JD, Dowell SF, Jorgensen JH, et al and the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Management of community-acquired pneumonia in the era of pneumococcal resistance. Arch Intern Med 2000; 160: 1399-1408.
- 24. Klugman KP, Madhi SA. Emergence of drug resistance. Impact on bacterial meningitis. Infect Dis Clin North Am 1999; 13: 637-646.
- 25. Turnidge JD, Bell JM, Collignon PJ. Rapidly emerging antimicrobial resistances in Streptococcus pneumoniae in Australia. Pneumococcal Study Group. Med J Aust 1999; 170: 152-155.
- 26. Therapeutic Guidelines Limited. Therapeutic guidelines: antibiotic. Version 11, 2000. Therapeutic Guidelines Limited, 2000.
- 27. Dobbin CJ, Duggan CJ, Barnes DJ. The efficacy of an antibiotic protocol for community-acquired pneumonia. Med J Aust 2001; 174: 333-337.
- 28. Andresen DN, Collignon PJ. Antibiotics for community-acquired pneumonia: time to return to the straight and narrow? Med J Aust 2001; 174: 321-322.
- 29. The Australian immunisation handbook. 7th ed. Canberra: National Health and Medical Research Council, 2000.





Abstract
Community-acquired pneumonia is caused by a range of organisms, most commonly Streptococcus pneumoniae, Mycoplasma pneumoniae, Chlamydia pneumoniae and respiratory viruses.
Chest x-ray is required for diagnosis.
A risk score based on patient age, coexisting illness, physical signs and results of investigations can aid management decisions.
Patients at low risk can usually be managed with oral antibiotics at home, while those at higher risk should be further assessed, and may need admission to hospital and intravenous therapy.
For S. pneumoniae infection, amoxycillin is the recommended oral drug, while benzylpenicillin is recommended for intravenous use; all patients should also receive a tetracycline (eg, doxycycline) or macrolide (eg, roxithromycin) as part of initial therapy.
Flucloxacillin or dicloxacillin should be added if staphylococcal pneumonia is suspected, and gentamicin or other specific therapy if gram-negative pneumonia is suspected; a third-generation cephalosporin plus intravenous erythromycin is recommended as initial therapy for severe cases.
Infections that require special therapy should be considered (eg, tuberculosis, melioidosis, Legionella, Acinetobacter baumanii and Pneumocystis carinii infection).