Up to 50% of antimicrobial agents prescribed to hospital inpatients are considered to be inappropriate,1,2 and this excess use has been associated with increased mortality, adverse drug reactions and the development of resistant bacteria.3,4 The Australian Commission on Safety and Quality in Health Care recently published recommendations for hospital-based antimicrobial stewardship programs.2 A variety of approaches are available to implement these recommendations, including dissemination of guidelines, education, restricting antimicrobial availability and postprescribing audit and review.
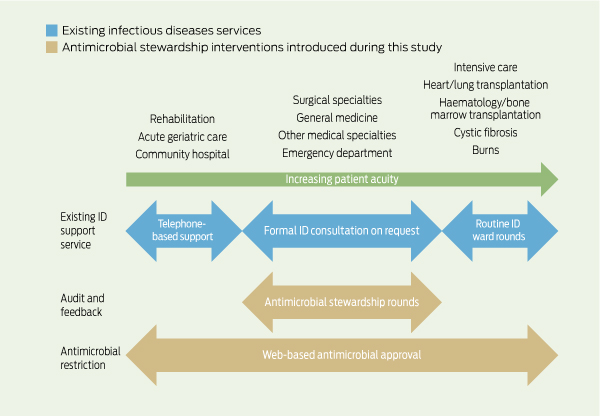
We have previously described the preliminary activities of the antimicrobial stewardship team.5 A web-based antimicrobial approval system (Guidance MS, Melbourne Health) was rolled out from October 20106 and a full-time pharmacist was appointed in January 2011. Before this, authorisation to prescribe restricted antimicrobial agents required approval from infectious diseases (ID) registrars, but auditing had suggested poor compliance. In the new system, online approval could be obtained to use restricted antimicrobials for pre-approved indications that were included in national or local consensus guidelines. Short-term approval was granted for other indications specified by the clinician (non-standard indications). Pharmacists could alert the antimicrobial stewardship team of unauthorised antimicrobial use exceeding 24 hours (pharmacist alerts).
Patients were reviewed by the stewardship team if they were receiving at least one restricted antimicrobial for a non-standard indication, where approval had expired, or where a pharmacist alert had been created. At our hospital, 13 restricted antimicrobial agents require web-based approval: amikacin, azithromycin, cefepime, ceftazidime, ceftriaxone, ciprofloxacin, meropenem, moxifloxacin, piperacillin/tazobactam, teicoplanin, ticarcillin/clavulanate, tobramycin and vancomycin. Patients were not reviewed by the antimicrobial stewardship team if they had already received a formal ID consult, or were admitted under lung transplant/cystic fibrosis, haematology and bone marrow transplant, or burns services, where ID physicians performed regular ward rounds (Box 1).
We compared trends in the rate of use of antimicrobial classes before stewardship implementation (January 2008 to December 2010) and after implementation (January 2011 to June 2012). Antimicrobial consumption quantities were converted into defined daily doses (DDD) per 1000 occupied bed-days (OBD) as part of the National Antimicrobial Utilisation Surveillance Program.7,8 Total broad-spectrum antimicrobial use was defined as the sum of usage for all classes except for aminoglycosides, which are regarded as narrow-spectrum antibiotics. Antimicrobial use is based on pharmacy purchasing data and inpatient stock distribution (excluding hospital in the home and the emergency department). Outcomes were assessed by:
the mean rate of antimicrobial use in the intervention period compared with the pre-intervention period;
model-predicted immediate change in antimicrobial use between the end of the pre-intervention period and the commencement of the intervention period (immediate change);
model-predicted change in the rate of antimicrobial use between the pre-intervention period and post-intervention period (change in trend);
the immediate change and the change in trend in antimicrobial use were both assessed using segmented Poisson regression.
We defined a clinically significant decrease in antimicrobial use as:
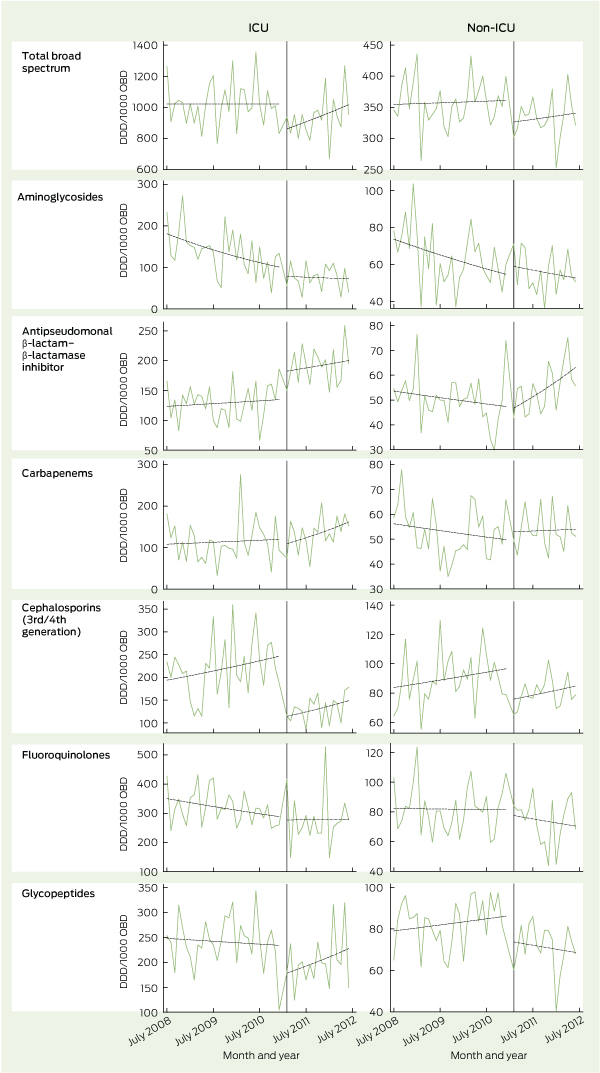
In the ICU, total broad-spectrum antimicrobial use decreased immediately by 16.6% when the intervention commenced (P < 0.001) (Box 2). The mean total use of broad-spectrum antimicrobials fell from 1022 DDD/1000 OBD in the pre-intervention period to 937 DDD/1000 OBD in the post-intervention period. Before the intervention, the rate of broad-spectrum antimicrobial use did not change; following the intervention, it increased by 1.0% per month (P < 0.001). Changes in the use of specific classes of antimicrobials are detailed in Box 2 and Box 3.
In hospital wards other than the ICU, total broad-spectrum antimicrobial use decreased by 9.9% when the intervention commenced (P = 0.002). The mean total use of broad-spectrum antimicrobials fell from 358 DDD/1000 OBD in the pre-intervention period to 333 DDD/1000 OBD in the post-intervention period. Before the intervention, the rate of broad-spectrum antimicrobial use increased by 0.1% per month; following the intervention, it increased by 0.3% per month (P = 0.49). Changes in the use of specific classes of antimicrobials are detailed in Box 2 and Box 3.
The antimicrobial stewardship program brought immediate reductions in the use of total broad-spectrum antimicrobials, particularly third/fourth generation cephalosporins and glycopeptides. In addition to case-by-case audit and feedback, regular stewardship rounds identified unapproved unit guidelines, provided an accessible clinical resource for junior doctors, raised awareness of appropriate antimicrobial use and reinforced the use of the web-based antimicrobial approval system. Our experience is consistent with a systematic review of stewardship programs that suggested that restrictive interventions were more likely to be successful than those based only on education or persuasion.9
The interventions that we have implemented are resource intensive, requiring a full-time pharmacist supported by part-time ID physicians (8–10 hours/week). Although a previous study has shown a decrease in several classes of broad-spectrum antimicrobials associated with a web-based approval system only,6 we felt that without an audit and feedback mechanism, this intervention would not be sustainable. Additionally, postprescribing audit and feedback recognises that appropriateness of therapy often needs to be considered on a case-by-case basis, and that broad guidelines on prescribing may not be easily applied to individual patients. Previous studies of similar interventions have found similar patterns of intervention, but on a much less intensive scale.10-12 Despite this, only six of 78 respondents in an Australian survey of hospital pharmacies reported implementing regular multidisciplinary antimicrobial stewardship ward rounds.13
1 Existing infectious diseases services and antimicrobial stewardship interventions introduced during the study
2 Change in antimicrobial use before and after implementation of antimicrobial stewardship interventions
Received 16 November 2012, accepted 17 February 2013
- Kelly A Cairns1
- Adam W J Jenney1,2
- Iain J Abbott1
- Matthew J Skinner1,3
- Joseph S Doyle1
- Michael Dooley1,2
- Allen C Cheng1,2
- 1 Alfred Health, Melbourne, VIC.
- 2 Monash University, Melbourne, VIC.
- 3 Sir Charles Gairdner Hospital, Perth, WA.
We acknowledge the previous antimicrobial stewardship pharmacists involved in this project (Jenny Kirschner, Trent Lee) and the members of the Alfred Health Antimicrobial Stewardship Committee. We also thank Alex Padiglione for helpful comments on the manuscript, and the National Antibiotic Utilisation Surveillance Program for data.
No relevant disclosures.
- 1. Gottlieb T, Nimmo GR. Antibiotic resistance is an emerging threat to public health: an urgent call to action at the Antimicrobial Resistance Summit 2011. Med J Aust 2011; 194: 281-283. <MJA full text>
- 2. Duguid M, Cruickshank M. Antimicrobial stewardship in Australian hospitals. Sydney: Australian Commission on Safety and Quality in Healthcare, 2011.
- 3. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44: 159-177.
- 4. Gums JG, Yancey RW Jr, Hamilton CA, Kubilis PS. A randomized, prospective study measuring outcomes after antibiotic therapy intervention by a multidisciplinary consult team. Pharmacotherapy 1999; 19: 1369-1377.
- 5. Cairns KA, Jenney AWJ, Krishnaswamy S, et al. Early experience with antimicrobial stewardship ward rounds at a tertiary referral hospital [letter]. Med J Aust 2012; 196: 34-35. <MJA full text>
- 6. Buising KL, Thursky KA, Robertson MB, et al. Electronic antibiotic stewardship — reduced consumption of broad-spectrum antibiotics using a computerized antimicrobial approval system in a hospital setting. J Antimicrob Chemother 2008; 62: 608-616.
- 7. Natsch S, Hekster YA, de Jong R, et al. Application of the ATC/DDD methodology to monitor antibiotic drug use. Eur J Clin Microbiol Infect Dis 1998; 17: 20-24.
- 8. South Australian Infection Control Service. National Antimicrobial Utilisation Surveillance Program: annual report 2010-2012. http://www.health.sa.gov.au/INFECTIONCONTROL/Default.aspx?PageContentID=65&tabid=199 (accessed Nov 2012).
- 9. Davey P, Brown E, Fenelon L, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2005; (4): CD003543.
- 10. Baker CA, Sehdev P. Defining an antimicrobial monitoring service. Am J Health Syst Pharm 2008; 65: 2095-2096, 2098-2099.
- 11. Laible BR, Nazir J, Assimacopoulos AP, Schut J. Implementation of a pharmacist-led antimicrobial management team in a community teaching hospital: use of pharmacy residents and pharmacy students in a prospective audit and feedback approach. J Pharm Pract 2010; 23: 531-535.
- 12. Cosgrove SE, Seo SK, Bolon MK, et al. Evaluation of postprescription review and feedback as a method of promoting rational antimicrobial use: a multicenter intervention. Infect Control Hosp Epidemiol 2012; 33: 374-380.
- 13. Chen AWJ, Khumra S, Eaton V, Kong DCM. Snapshot of antimicrobial stewardship in Australian hospitals. J Pharm Prac Res 2010; 40: 19-26.
- 14. Peterson LR. Squeezing the antibiotic balloon: the impact of antimicrobial classes on emerging resistance. Clin Microbiol Infect 2005; 11 Suppl 5: 4-16.





Abstract
Objectives: Antimicrobial stewardship programs are recommended to reduce antimicrobial resistance by reducing inappropriate use of antimicrobials. We implemented an antimicrobial stewardship program and aimed to evaluate its effect on broad-spectrum antimicrobial use.
Design, setting and participants: Observational study with historical control using interrupted time series analysis conducted in a tertiary referral hospital. Hospital inpatients prescribed restricted antimicrobials for non-standard indications, where approval had expired or without approval.
Intervention: Baseline period of 30 months immediately followed by an 18-month intervention period commencing January 2011.
Main outcome measures: Number and type of interventions made by antimicrobial stewardship team; monthly rate of use of broad-spectrum antimicrobial agents (in defined daily doses/1000 occupied bed-days).
Results: The antimicrobial stewardship team made 1104 recommendations in 779 patients during the 18-month intervention period. In 64% of cases, the recommendation was made to cease or de-escalate the antimicrobial therapy, or to change from intravenous to oral therapy. The introduction of the intervention resulted in an immediate 17% (95% CI, 13%–20%) reduction in broad-spectrum antimicrobial use in the intensive care unit and a 10% (95% CI, 4%–16%) reduction in broad-spectrum antimicrobial use outside the intensive care unit. Reductions were particularly seen in cephalosporin and glycopeptide use, although these were partially offset by increases in the use of β-lactam–β-lactamase inhibitors.
Conclusions: The introduction of an antimicrobial stewardship program, including postprescription review, resulted in an immediate reduction in broad-spectrum antimicrobial use in a tertiary referral centre. However, the effect of this intervention reduced over time.