Chronic obstructive pulmonary disease (COPD) is a major public health problem. It is the single leading cause of death that is continuing to increase and is now the third largest contributor to the burden of disease in Australia.1 In response to this challenge, the Australian Lung Foundation and Thoracic Society of Australia and New Zealand developed clinical practice guidelines (COPDX) to improve the diagnosis and management of COPD. These guidelines were published 3 years ago as a supplement to the Journal2 and were based largely on evidence in the Global Initiative for Chronic Obstructive Lung Disease (GOLD).3 Since then, the Australian Lung Foundation has updated COPDX, incorporating more recently published evidence and systematic reviews in the Cochrane Library. Levels of evidence have been reclassified for this summary in accordance with guidelines from the National Health and Medical Research Council (NHMRC).4
Spirometry remains the mainstay of diagnosis of COPD, but is underutilised in Australia. Trials are currently underway to explore barriers to the use of spirometry in general practice, to develop simple reliable questionnaires that may guide general practitioners in screening patients for referral, and to confirm that more widespread application improves outcomes in chronic respiratory diseases. A diagnostic algorithm is under evaluation on the Australian Lung Foundation website (http://www.lungnet.org.au) (Box 1).
Long-acting β2 agonists: Regular treatment with long-acting β2 agonists is more effective and convenient than treatment with short-acting bronchodilators (evidence level I) and is associated with improved quality of life5 (evidence level II). However, a systematic review of eight randomised controlled trials (RCTs) of long-acting β2 agonists found no overall difference in forced expiratory volume in 1 second (FEV1) when compared with placebo (evidence level I), and only one trial reported less dyspnoea in patients during treatment with these drugs6 (evidence level II). As the review excluded patients with > 15% reversibility after a dose of short-acting β2 agonist, it may underestimate the benefits of long-acting β2 agonists in unselected COPD patients.
Tiotropium: This inhaled anticholinergic agent has a duration of effect longer than 24 hours, and so can be taken once daily.7 Compared with placebo and regular ipratropium, tiotropium reduces exacerbations and improves quality of life.8 It also decreases exertional dyspnoea and increases endurance by reducing hyperinflation9 (evidence level II).
Combination inhalers: Combinations of an inhaled glucocorticoid and long-acting β2 agonist in a single inhaler are being increasingly used in COPD. A systematic review of six RCTs of combination inhalers for COPD concluded that, compared with placebo, combination therapy led to clinically meaningful differences in quality of life, symptoms and frequency of exacerbations10 (evidence level I). However, comparison of the different combination therapies with their single components gave conflicting results, possibly because of differential drop-out rates in the original studies (Professor Paul Jones, St Georges Hospital, London, personal communication). Again, patients with > 12% and 200 mL bronchodilator reversibility were excluded from four of the six trials in this review, potentially reducing the benefits that might be seen in a more general population with COPD. Firmer conclusions about the effects of combination therapy in a single inhaler require more data, including comparison with the effects of the two drugs administered separately in double-dummy trials.
Oral glucocorticoids: The use of oral glucocorticoids in stable COPD was recently examined in a systematic review of 24 RCTs.11 Overall, it would be necessary to treat seven patients (95% CI, 5–12) with oral steroids to achieve one extra case of an increase in FEV1 greater than 20%. There was no evidence to support the long-term use of oral steroids at daily doses less than 10–15 mg prednisolone, although some evidence that higher doses (≥ 30 mg) improved lung function over a short period. Potentially harmful adverse effects would prevent us recommending long-term use at these high doses in most patients (evidence level I).
In younger patients with asthma or mild COPD, there is no evidence of an effect of inhaled glucocorticoids in daily doses of 1000 μg or less of fluticasone or equivalent given for 2–3 years on bone mineral density (BMD) or vertebral fractures12 (evidence level I). Higher doses are associated with biochemical markers of increased bone turnover, but data on BMD and fractures at these doses are not yet available. In older people, the BMD and fracture safety profile of most inhaled glucocorticoids for the treatment of COPD is not known. However, triamcinolone was associated with reduced BMD in the Lung Health Study13 (evidence level II). Australian guidelines have been published on the prevention and treatment of osteoporosis, including glucocorticoid-induced osteoporosis.14
Non-invasive positive pressure ventilation: Although 12 to 24-month studies in patients with stable COPD with chronic respiratory failure have suggested that the addition of non-invasive positive pressure ventilation (NPPV) may have some beneficial effects,15,16 its widespread use cannot yet be advocated.17 Compared with long-term oxygen therapy alone, the addition of NPPV has some beneficial effects on CO2 retention and shortness of breath.16 A well powered RCT is near completion in Australia, evaluating NPPV use in COPD patients with hypercapnia.
Pulmonary rehabilitation: This reduces dyspnoea, anxiety and depression, improves exercise capacity and quality of life, and may reduce hospitalisation (evidence level I). The minimum duration of an effective rehabilitation program that includes exercise training is 6 weeks; the longer the program continues, the more effective it appears to be18-20 (evidence level II). However, as yet, effective structures that maintain benefit have not been subjected to robust clinical trials.21
Anabolic steroids and nutritional supplements: In patients with COPD and weight loss, anabolic steroids may increase body weight and lean body mass, but have little or no effect on exercise capacity22,23 (evidence level II). Although weight loss and low body mass index are both poor prognostic indicators in COPD, there is level I evidence that nutritional supplementation does not alter anthropometric measures, lung function or exercise capacity when nutrition is already depleted.24
Opioids: These may have a role in the relief of severe intractable dyspnoea25,26 (evidence level I).
The mainstay for preventing deterioration in COPD is complete cessation of smoking, as only complete cessation slows decline in lung function.27 A combination of psychosocial and pharmacological interventions is superior to no treatment or psychosocial interventions alone for achieving smoking cessation28 (evidence level I).
Recommended vaccinations in COPDX have been harmonised with NHMRC approved guidelines (http://immunise.health.gov.au/handbook.htm). In addition, there is some evidence of a possible benefit from Haemophilus influenzae vaccination. Six RCTs of oral killed non-typable H. influenzae vaccine found a significant reduction in the incidence of bronchitic episodes 3 months after vaccination, but the effect had disappeared by 9 months.29 The severity of exacerbations in the treatment group, as measured by the requirement to prescribe antibiotics, was reduced by 65% at 6 months (evidence level I). However, a larger clinical trial is needed to assess longer term prognosis, and the vaccine is not currently available. Another systematic review of 13, mostly low-quality, trials of oral lyophilised bacterial extracts in COPD found some evidence of symptomatic improvement, but no convincing reduction in exacerbations30 (evidence level I).
The effect of inhaled glucocorticoids on decline in lung function is controversial. Systematic reviews and meta-analyses of the available RCTs have found a small benefit of uncertain significance when compared with placebo (evidence level I). A 2003 meta-analysis found a combined difference in the rate of decline in FEV1 of 5.0 mL/year between treatment groups (95% CI, − 1.2 to 11.2 mL/year),31 while a second meta-analysis in the same year found a combined difference of 7.7 mL/year (95% CI, 1.3–14.2 mL/year).32 The varying conclusions of these reviews would not lead to a recommendation for inhaled glucocorticoids to be used routinely in all patients with COPD. However, these drugs are indicated for patients with a previously documented response and for those who have severe COPD with frequent exacerbations (evidence level II).
Mucolytic agents may reduce the frequency and duration of exacerbations (evidence level I). A systematic review concluded that, in patients with COPD or chronic bronchitis who have a higher than average rate of exacerbations, treatment with mucolytic agents was associated with a small but significant reduction in acute exacerbations and total days of disability.33 However, a recent large RCT of N-acetylcysteine did not confirm an overall reduction in exacerbations, although a significant reduction was still seen in the subgroup who were not having concomitant treatment with inhaled steroids34 (evidence level II). Nonetheless, such agents are not available for COPD through the Pharmaceutical Benefits Scheme (PBS) or Repatriation PBS, and are thus currently not widely used in Australia.
Patients should be encouraged to take appropriate responsibility for their own management (evidence level III-1). However, a systematic review of self-management plans in COPD found no effect on hospital admissions, emergency department visits, days lost from work or lung function.35 Inconclusive results were observed for health-related quality of life, COPD symptoms and use of other health care resources. Clearly, further well designed RCTs with sufficiently long follow-up are required. Nonetheless, self-management education reduced the need for rescue medication and led to increased use of oral steroids and antibiotics for respiratory symptoms (evidence level I).
Hospital in the home: Up to a quarter of carefully selected patients presenting to hospital emergency departments with acute exacerbations of COPD can be safely and successfully treated at home with support from respiratory nurses. A systematic review of seven RCTs of “hospital in the home” schemes found no significant differences in readmission rates or mortality, and a preference for these schemes by patients and carers36 (evidence level I). However, further research is needed, as the studies reviewed were small and varied in the interventions used.
Nebulised β 2 agonists and anticholinergics: Hospital management of a severe exacerbation of COPD usually includes nebulised β2 agonist, administered continuously in extremely unwell patients and intermittently in others. An anticholinergic agent may be delivered together with the nebulised β2 agonist in patients with severe exacerbations or when response to the β2 agonist alone is poor. However, a systematic review that included four RCTs did not demonstrate any additional benefit on FEV1 of combining an anticholinergic compared with β2 agonists alone37 (evidence level I).
Aminophylline: The routine use of intravenous aminophylline is no longer recommended for acute exacerbations of COPD (evidence level I). A systematic review of four RCTs of methylxanthines found only a transient increase of 101 mL in FEV1 after 3 days, with a 4.6-fold increased risk of nausea and vomiting.38 This is confirmed by a recent RCT in patients with non-acidotic acute exacerbations, which found no clinically useful reductions in breathlessness or length of hospital stay and no improvement in lung function, but significantly more nausea among those treated with aminophylline.39
Antibiotic therapy: Exacerbations with clinical signs of infection benefit from antibiotic therapy. A recent multicentre RCT found that moxifloxacin was equivalent to standard antibiotics (amoxycillin, clarithromycin or cefuroxime) for clinical success, and superior for clinical cure and bacteriological eradication, and reduced the frequency of exacerbations over the following 5 months.40 These findings applied to patients with milder COPD, most of whom were not prescribed oral steroids (evidence level II).
Non-invasive positive pressure ventilation: NPPV is effective for managing acute hypercapnic ventilatory failure in COPD (evidence level I). A systematic review of 14 RCTs found that NPPV resulted in significantly decreased mortality, decreased need for endotracheal intubation, and more rapid improvement in arterial blood gases.41 Length of hospital stay was reduced by a mean of 3.2 days. When intubation is required, weaning from ventilation is facilitated by NPPV. A systematic review of five RCTs found that the NPPV-weaning strategy was associated with significantly lower mortality and reduced length of hospital stay by a mean of 7.3 days.42
The evidence base for safe and effective management of COPD continues to improve, although there is still a need for well designed RCTs, particularly of non-pharmacological interventions. Further evidence from RCTs and systematic reviews needs to be couched in terms of meaningful outcomes and should provide the numbers needed to treat for benefit and harm. It also needs to be remembered that absence of evidence for a treatment effect is not the same as evidence for absence of an effect. The challenge for the Australian Lung Foundation and the Thoracic Society of Australia and New Zealand is to disseminate COPDX efficiently and to improve the diagnosis and management of COPD in Australia. The most recent approved full version of the guidelines is available at <http://www.copdx.org.au>.
1 Diagnostic algorithm to distinguish chronic obstructive pulmonary disease (COPD) from asthma
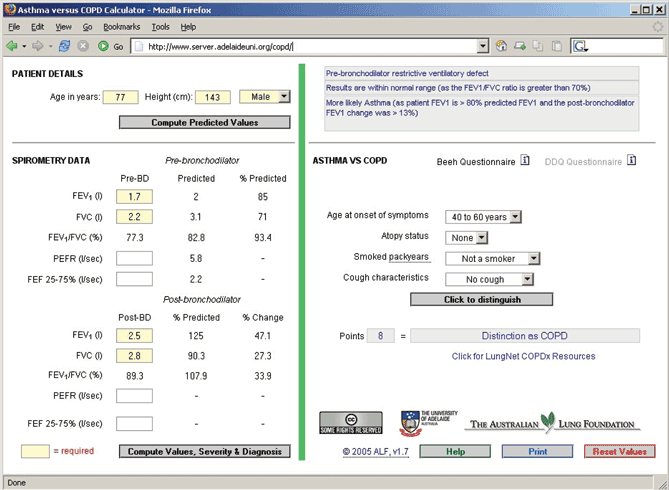
The patient’s age, height, sex and spirometry results are entered in the left column. A brief questionnaire is completed in the right hand column. The program calculates predicted values and severity, and provides a likely diagnosis. (Available at: http://www.lungnet.org.au.)
2 Therapies to optimise function and prevent deterioration in chronic obstructive pulmonary disease
To prevent deterioration | |||||||||||||||
- Michael J Abramson1
- Alan J Crockett2
- Peter A Frith3
- Christine F McDonald4
- 1 Department of Epidemiology and Preventive Medicine, Monash University, Melbourne, VIC.
- 2 Discipline of General Practice, University of Adelaide, Adelaide, SA.
- 3 Respiratory Department, Repatriation General Hospital, Adelaide, SA.
- 4 Department of Respiratory and Sleep Medicine, Austin Health, Melbourne, VIC.
The Australian Lung Foundation supported meetings of authors and preparation of this article. We also wish to thank the other members of the COPD Evaluation Committee, Nicholas Glasgow, Susan Jenkins and Richard Wood-Baker, for their helpful comments.
Michael Abramson has served on the scientific advisory committee for the Australian Asthma Study, which was sponsored by GlaxoSmithKline, and received travel assistance from AstraZeneca on one occasion.
Alan Crockett has received fees from several pharmaceutical companies for providing training in spirometry for general practitioners and practice nurses.
Peter Frith has received honoraria from Boehringer Ingelheim, Pfizer, GlaxoSmithKline and AstraZeneca for delivering lectures and workshops to general practitioners on the use of COPDX, and a grant-in-aid from AltanaPharma for travel to the Thoracic Society of Australia and New Zealand annual scientific meeting.
Christine McDonald has received honoraria for speaking at meetings sponsored by Boehringer Ingelheim, Pfizer, GlaxoSmithKline and AstraZeneca, and travel assistance from these companies to attend international respiratory meetings.
- 1. Mathers C, Vos T, Stevenson C. The burden of disease and injury in Australia: summary report. Canberra: Australian Institute of Health and Welfare, 1999.
- 2. McKenzie DK, Frith PA, Burdon JGW, Town GI. The COPDX Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2003. Med J Aust 2003; 178 (6 Suppl): S1-S40. <MJA full text>
- 3. Pauwels RA, Buist AS, Calverley PMA, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001; 163: 1256-1276.
- 4. National Health and Medical Research Council. A guide to the development, implementation and evaluation of clinical practice guidelines. Canberra: NHMRC, 1999. Available at: http://www.nhmrc.gov.au/publications/synopses/cp30syn.htm (accessed Jan 2006).
- 5. Dahl R, Greefhorst LA, Nowak D, et al. Inhaled formoterol dry powder versus ipratropium bromide in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 778-784.
- 6. Appleton S, Poole P, Smith B, et al. Long-acting beta2-agonists for chronic obstructive pulmonary disease patients with poorly reversible airflow limitation. Cochrane Database Syst Rev 2001; 4: CD001104.
- 7. Casuburi R, Mahler DA, Jones PW, et al. A long term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19: 217-224.
- 8. Vincken W, van Noord JA, Greefhorst AP, et al. Improved health outcomes in patients with COPD during 1 year’s treatment with tiotropium. Eur Respir J 2002; 19: 209-216.
- 9. O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23: 825-831.
- 10. Nannini L, Cates CJ, Lasserson TJ, et al. Combined corticosteroid and longacting beta-agonist in one inhaler for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004; 3: CD003794.
- 11. Walters JA, Walters EH, Wood-Baker R. Oral corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005; 3: CD005374.
- 12. Jones A, Fay JK, Burr M, et al. Inhaled corticosteroid effects on bone metabolism in asthma and mild chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; 1: CD003537.
- 13. Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000; 343: 1902-1909.
- 14. Sambrook PN, Seeman E, Phillips SR, Ebeling PR. Preventing osteoporosis: outcomes of the Australian Fracture Prevention Summit. Med J Aust 2002; 176 (8 Suppl): 1-16. <MJA full text>
- 15. Casanova F, Celli BR, Tost L, et al. Long-term controlled trial of nocturnal nasal positive pressure ventilation in patients with severe COPD. Chest 2000; 118: 1582-1590.
- 16. Clini E, Sturani C, Rossi A, et al. The Italian multicentre study on non-invasive ventilation in chronic obstructive pulmonary disease patients. Eur Respir J 2002; 20: 529-538.
- 17. Elliott MW. Noninvasive ventilation in chronic ventilatory failure due to chronic obstructive pulmonary disease. Eur Respir J 2002; 20: 511-514.
- 18. Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000; 355: 362-368.
- 19. Finnerty JP, Keeping I, Bullough I, et al. The effectiveness of outpatient pulmonary rehabilitation in chronic lung disease; a randomised controlled trial. Chest 2001; 119: 1705-1710.
- 20. Green RH, Singh SJ, Williams J, et al. A randomised controlled trial of four weeks versus seven weeks of pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax 2001; 56: 143-145.
- 21. Ries AL, Kaplan RM, Myers R, et al. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomised trial. Am J Respir Crit Care Med 2003; 167: 880-888.
- 22. Yeh SS, DeGuzman B, Kramer T, et al. Reversal of COPD-associated weight loss using the anabolic agent oxandrolone. Chest 2002; 122: 421-428.
- 23. Weisberg J, Wanger J, Olson J, et al. Megestrol acetate stimulates weight gain and ventilation in underweight COPD patients. Chest 2002; 121: 1070-1078.
- 24. Ferreira IM, Brooks D, Lacasse Y, et al. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005; 2: CD000998.
- 25. Jennings A-L, Davies AN, Higgins JPT, et al. A systematic review of the use of opioids in the management of dyspnoea. Thorax 2002; 57: 939-944.
- 26. Abernethy AP, Currow DC, Frith P, et al. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ 2003; 327: 523-528.
- 27. Simmons MS, Connett JE, Nides MA, et al. Smoking reduction and the rate of decline in FEV1: results from the Lung Health Study. Eur Respir J 2005; 25: 1011-1017.
- 28. van der Meer RM, Wagena EJ, Ostelo RW, et al. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2001; 1: CD002999.
- 29. Foxwell AR, Cripps AW, Dear KBG. Haemophilus influenzae oral whole cell vaccination for preventing acute exacerbations of chronic bronchitis. Cochrane Database Syst Rev 2003; 3: CD001958.
- 30. Steurer-Stey C, Bachmann LM, Steurer J, et al. Oral purified bacterial extracts in chronic bronchitis and COPD: systematic review. Chest 2004; 126: 1645-1655.
- 31. Highland KB, Strange C, Heffner JE. Long-term effects of inhaled corticosteroids on FEV1 in patients with chronic obstructive pulmonary disease: a meta analysis. Ann Intern Med 2003; 138: 969-973.
- 32. Sutherland ER, Allmers H, Ayas NT, et al. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: a meta-analysis. Thorax 2003; 58: 937-941.
- 33. Poole PJ, Black PN. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003; 1: CD001287.
- 34. Decramer M, Rutten-van Molken M, Dekhuijzen PN, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 2005; 365: 1552-1560.
- 35. Monninkhof EM, van der Valk PD, van der Palen J, et al. Self-management education for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2002; 4: CD002990.
- 36. Ram FSF, Wedzicha JA, Wright J, et al. Hospital at home for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003; 4: CD003573.
- 37. McCrory DC, Brown CD. Anti-cholinergic bronchodilators versus beta2-sympathomimetic agents for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003; 1: CD003900.
- 38. Barr RG, Rowe BH, Camargo CAJ. Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003; 2: CD002168.
- 39. Duffy N, Walker P, Diamantea F, et al. Intravenous aminophylline in patients admitted to hospital wih exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Thorax 2005; 60: 713-717.
- 40. Wilson R, Allegra L, Huchon G, et al. Short-term and long-term outcomes of moxifloxacin compared with standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 2004; 125: 953-964.
- 41. Ram FSF, Picot J, Lightowler J, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004; 3: CD004104.
- 42. Burns KEA, Adhikari NKJ, Meade MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev 2003; 4: CD004127.





Abstract
Long-acting β2 agonists are an effective and convenient treatment for chronic obstructive pulmonary disease (COPD), but do not significantly improve lung function.
The long-acting anticholinergic tiotropium, which can be taken once daily, decreases exertional dyspnoea and increases endurance by reducing hyperinflation.
The role in COPD of the combination of a long-acting β2 agonist and a glucocorticoid in a single inhaler remains unclear.
The minimum duration of an effective pulmonary rehabilitation program that includes exercise training is 6 weeks.
Long-term treatment with inhaled glucocorticoids may reduce the rate of decline in lung function, but the effect is small.
Aminophylline should no longer be routinely used in acute exacerbations of COPD.
Non-invasive positive pressure ventilation (NPPV) reduces mortality and hospital stay in patients with acute hypercapnic ventilatory failure; it is also an effective weaning strategy for patients who require intubation.
Further studies are required to clarify the role of NPPV in the long-term management of stable COPD.