Transfusion of allogenic packed red blood cells (RBC) is common in intensive care units (ICU), but prospective epidemiological data, both national and international, are lacking. In one retrospective study, more than 70% of patients with a length of stay in an intensive care unit of longer than one week received an RBC transfusion,1 and in a study examining ICU transfusion practice and mortality a third of all ICU admissions received an RBC transfusion.2
Concerns about the rate of inappropriate transfusion exist, particularly given the recognised risks of transfusion3 and the decreasing availability of donor blood.4 Previous studies of RBC transfusion in Australian hospitals have found rates of inappropriate transfusion between 16% and 30%.5-7
We conducted a prospective, multicentre, observational study to determine the incidence, indications and appropriateness of RBC transfusions in Australasian intensive care practice.
We sent an invitation to participate in the study to all 35 units affiliated with the Australian and New Zealand Intensive Care Society Clinical Trials Group.
Institutional ethics committee approval was either obtained or waived according to local regulations for audit procedure. Each participating unit screened all ICU admissions during March 2001.
Data were collected on all patients who received an RBC transfusion during their ICU stay. Data collection was modelled on the Anaemia and Blood Transfusion in Critical Care Survey.8 For each patient transfused with RBC during their ICU admission, we collected demographic data, admission type, diagnostic category, Acute Physiology and Chronic Health Evaluation (APACHE) II score,9 comorbidities, and peripheral blood haemoglobin level at admission. For each transfusion day (see Box 1), the indication for transfusion, pre-transfusion haemoglobin levels, and number of units transfused were recorded. Patients were followed until Day 28 or hospital discharge, transfer or death if this occurred earlier. For the complete study period, all measured haemoglobin levels were recorded and all transfusion episodes, including those occurring after ICU discharge but within the study period, were documented. To determine appropriateness, each transfusion episode was reviewed against the indications of the National Health and Medical Research Council (NHMRC) clinical practice guidelines for appropriate use of red blood cells (Box 2).10 Documentation of "diminished physiological reserve" as the indication for transfusion was accepted as appropriate if the pre-transfusion haemoglobin level was < 100 g/L. ICU research coordinators either transcribed data to a case report form or entered data directly into an Access 97 database.11 Data were reviewed for inconsistencies or data entry errors by one of the authors (C J F). After data verification, individual hospital databases were combined.
Statistical analysis was performed using Analyse-it.12 Data were tested for normality using the Kolmogorov–Smirnov test. For non-normally distributed data, the Mann–Whitney U test was used for comparison of continuous variables in two unrelated groups, the χ2 test for categorical data, and the H test (Kruskal–Wallis) for continuous variables in three or more unrelated groups. Spearman's rank order coefficient was calculated to determine correlation between continuous variables. A P value of 0.05 or less was considered significant.
Eighteen (51%) of the 35 ICUs participated. Sixteen units were in Australia and two in New Zealand. Fifteen were metropolitan tertiary referral units, two were major regional units and one a metropolitan private unit.
During the study period, there were 1808 ICU admissions and 357 (350 patients) received an RBC transfusion (19.7% transfusion rate). Mortality for transfused patients was 17.1% (60/350). The characteristics of ICU admissions who received an RBC transfusion are summarised in Box 3. There were 663 transfusion days and 1631 units of RBC were administered. Of the 663 transfusion days, 576 (involving the administration of 1464 RBC units) occurred in the ICU. Further RBC transfusions were received by 15.4% (54/350) of patients after ICU discharge.
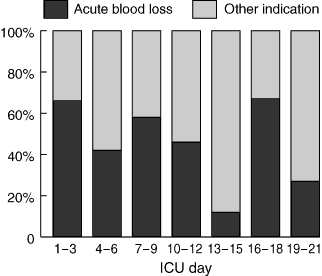
The indications for the transfusions are presented in Box 4. Acute bleeding was the indication in more than 60% (652/985) of transfusions in the first 72 hours of ICU admission, with non-acute indications accounting for more than 60% (285/470) of transfusions after this period (Box 5). In 3% (44/1464) of transfusions (22 patients), the indication for RBC did not comply with the NHMRC guidelines (Box 6). No admission type was associated with a higher incidence of inappropriate transfusion (P = 0.5).
Six of the RBC units that were administered after ICU discharge did not comply with NHMRC guidelines. Overall, 3.1% (50/1631) of RBC transfusions to patients admitted to participating ICUs in March 2001 were outside NHMRC guidelines.
We found that 19.7% of admissions to 18 self-selected Australian and New Zealand intensive care units received an RBC transfusion. There were 44 (3%) RBC units inappropriately transfused within the ICU during the study period. Limited Australian data exist on the overall proportion of RBC transfusions that occur in ICUs. In one study, ICU physicians administered 15% of all prescribed RBC transfusions; only nephrology and medical oncology units had higher rates.7 In 1995, the rate of inappropriate RBC transfusion in a large tertiary institution was found to be 10%.7 That single-centre study evaluated 200 transfusion episodes and 558 units of transfused RBCs in a 60-day period in 1993 and included both general ward and intensive care patients. More recently, in a retrospective audit, the rate of inappropriate transfusion in the same institution was 20%.5 In a multicentre study in metropolitan Sydney, the rate of inappropriate transfusion was 35%.6 In this latter study, ICU patients were excluded. Wide variation in the rate of inappropriate transfusion therefore appears to exist. As ICUs are large users of RBC, a high inappropriate transfusion rate would result in significant waste of a scarce resource.
Why then is the rate of inappropriate transfusion in our ICU patients so low? Previous studies5-7 examining transfusion practice in Australia were conducted in 1993–1999. There are no data as to the rate of inappropriate transfusion in intensive care units in this period. In a 1998 Canadian survey,13 marked variation in critical care transfusion practice was found, with many intensivists adhering to a historical 100 g/L threshold. This arbitrary level was maintained in the belief that improved oxygen delivery would attenuate organ dysfunction.14 In our study, however, the median pretransfusion haemoglobin level was 82 g/L. In a recent audit of European transfusion practice in intensive care, the median pretransfusion haemoglobin level was also 82 g/L (Professor J L Vincent, Erasmus University, Brussels, Belgium, personal communication).
In 1999, the Canadian Clinical Trials Group reported a multicentre randomised controlled trial evaluating transfusion requirements in critical care (TRICC).15 It was the first controlled study that suggested there is no clinical benefit in maintaining a haemoglobin level greater than 100 g/L in critically ill patients. It is possible that publication of the TRICC results significantly affected transfusion practice in Australasian intensive care.
A yet more aggressive indicator of appropriateness is to compare our practice with the TRICC restrictive strategy — a transfusion trigger of 70 g/L, with the haemoglobin level maintained at 70–90 g/L.15 The implementation of such a strategy, however, would be controversial. There is concern about critically ill patients with a cardiovascular diagnosis.16-18 In our study, in patients without a diagnosis of cardiovascular disease who received a transfusion, the haemoglobin level 24 hours after transfusion was greater than 90 g/L in 70% (340/469) of transfusion days. If the target post-transfusion haemoglobin level had been 90 g/L, then theoretically the transfusion of about 200 units of RBCs could have been avoided.
We have demonstrated that, compared with published guidelines, the rate of inappropriate transfusion in intensive care admissions is 3%. This rate of inappropriate transfusion is markedly lower than previously published data for non-critically ill patients. Despite this, if a more restrictive transfusion strategy for critically ill patients were implemented in Australia and New Zealand further significant reductions in RBC transfusion could be achieved. Although the reasons for the low inappropriate transfusion rate reported here are uncertain, it is possible that publication of the TRICC study15 has influenced intensive care practice. Other groups that prescribe large numbers of RBC transfusions should be encouraged to conduct similar studies to provide Level 1 evidence for transfusion thresholds in their patient populations.
* The ANZICS Clinical Trial Group
Participating centres and investigators: Austin and Repatriation Medical Centre, VIC: Rinaldo Bellomo, Donna Goldsmith; Cabrini Medical Centre, VIC: Felicity Hawker; Royal Canberra Hospital, ACT: Imogen Mitchell, Joy Whiting; Flinders Medical Centre, SA: Andrew Bersten, Tamara Hunt; Gold Coast Hospital, QLD: Brent Richards, Mandy Tallot; Hawkes Bay Hospital, New Zealand: Ross Freebairn; Middlemoore Hospital, New Zealand: Jane Clarke, Margaret Dewse; Princess Alexandra Hospital, QLD: Chris Joyce, Tony Limpus; Royal Adelaide Hospital, SA: Peter Sharley, Stephanie Creed; Royal Brisbane Hospital, QLD: Jeff Lipman, Bronwyn Couchman, Judith Perrott; Royal Hobart Hospital, TAS: Andrew Turner; Royal Melbourne Hospital, VIC: Megan Robertson, Cathy Boyce; Royal North Shore Hospital, NSW: Simon Finfer, Andrew Marich; Royal Perth Hospital, WA: Geoff Dobb, Teresa Williams; Sir Charles Gairdner Hospital, WA: Mary Pinder, Brigitte Roberts; St George Hospital, NSW: John Myburgh, Kathyrn Girling; Western Hospital, VIC: Craig French, Anna Green, Julie Daniels; Wollongong Hospital, NSW: Sundaram Rachakonda, EG Simmons.
1: Definitions
For this study the following definitions were used.
Anaemia: A haemoglobin level less than 100 g/L.
Transfusion day: A day on which a patient received an RBC transfusion.
Acute blood loss: Blood loss occurring within a 72-hour period that required transfusion or produced anaemia.
Altered tissue perfusion: Presence of cardiovascular organ dysfunction requiring intravenous inotrope use or elevated plasma lactate level.
Diminished physiological reserve: Transfusion in the absence of altered tissue perfusion or acute bleeding where the clinician believes there is benefit from RBC transfusion. In particular, the clinician believes that the patient is at high risk of developing clinical signs, symptoms or evidence of impaired oxygen transport. This definition was designed for use in the Anaemia and Blood Transfusion in Critical Care Survey.8
2: Summary of NHMRC guidelines for appropriate use of red blood cells10
The decision to transfuse red blood cells should be based on clinical assessment of the patient.
Use of red blood cells is likely to be inappropriate when the haemoglobin level is > 100 g/L, unless there are specific indications (Level I evidence). If red blood cells are given at this haemoglobin level, reasons should be well documented.
Use of red blood cells may be appropriate when the haemoglobin level is in the range 70–100 g/L (Level IV evidence). In such cases, the decision to transfuse should be supported by the need to relieve clinical signs and symptoms and prevent significant morbidity and mortality.
Use of red blood cells is likely to be appropriate when the haemoglobin level is < 70 g/L (Level IV evidence). In some patients who are asymptomatic and/or where specific therapy is available, lower threshold levels may be acceptable.
When the haemoglobin level is in the range 70–100 g/L, clinical judgement about the risk of transfusion is of great importance. Use of red blood cells may be appropriate (Level IV evidence) when:
the patient is undergoing an operative procedure associated with major blood loss;
there are clinical signs, symptoms or evidence that the patient has associated impairment in oxygen transport that may be exacerbated by anaemia;
to control anaemia-related symptoms in a patient on a chronic transfusion regimen or during marrow suppressive therapy and to maintain the haemoglobin level > 80 g/L.
3: Characteristics of all transfused admissions to 18 Australasian intensive care units (n = 357)
Median (range) patient age (years) |
68 |
(18–94) |
|||||||||
Median (range) APACHE II score |
16 |
(0–48) |
|||||||||
Sex (% male) |
64.7% |
(231/357) |
|||||||||
Mortality |
17.1% |
(60/350) |
|||||||||
Admission type |
|||||||||||
Elective surgery |
42.3% |
(151/357) |
|||||||||
Emergency surgery |
19.0% |
(68/357) |
|||||||||
Trauma |
13.7% |
(49/357) |
|||||||||
Medical |
25.0% |
(89/357) |
|||||||||
Primary admission category |
|||||||||||
Cardiovascular system |
36.1% |
(129/357) |
|||||||||
Respiratory |
9.5% |
(34/357) |
|||||||||
Trauma |
11.2% |
(40/357) |
|||||||||
Gastrointestinal tract |
16.8% |
(60/357) |
|||||||||
Hepatobiliary tract |
3.9% |
(14/357) |
|||||||||
Neurological |
6.7% |
(24/357) |
|||||||||
Other |
18.5% |
(66/357) |
|||||||||
Median (range) admission haemoglobin level (g/L) |
98 |
(44–214) |
|||||||||
Median (range) pre-transfusion haemoglobin level (g/L) |
82 |
(44–187) |
|||||||||
Median (range) post-transfusion haemoglobin level (g/L) |
98 |
(51–165) |
|||||||||
Transfusion days with post-transfusion haemogloblin level > 90 g/L |
70.8% |
(428/604) |
|||||||||
Red blood cell transfusions |
|||||||||||
1 unit |
154 |
||||||||||
2 units |
278 |
||||||||||
3 units |
70 |
||||||||||
4 units |
40 |
||||||||||
5 or more units |
54 |
||||||||||
Transfusions in the 24 hours before admission to intensive care unit |
37.8% |
(135/357) |
|||||||||
4: Indications for 1464 red blood cell transfusions in 18 Australasian intensive care units
Indication |
Number (%) |
Median (range) pre-transfusion |
|||||||||
Acute blood loss* |
880 |
(60.1%) |
82 (44–187) |
||||||||
Non-acute bleeding |
584 |
(39.9%) |
|||||||||
Diminished physiological reserve* |
423 |
(28.8%) |
79 (54–119) |
||||||||
Altered tissue perfusion* |
77 |
(5.3%) |
82 (44–118) |
||||||||
Coronary artery disease |
8 |
(0.4%) |
81 (78–94) |
||||||||
Indication not defined |
80 |
(5.4%) |
90 (53–129) |
||||||||
* For definitions, see Box 1. |
|||||||||||
6: Indications for 44 transfusions that were deemed inappropriate
Indication |
Number (%) |
Median (range) pre-transfusion |
|||||||||
Indication not defined |
15 (34%) |
109 (100–129) |
|||||||||
Diminished physiological reserve* |
22 (50%) |
103 (100–119) |
|||||||||
Altered tissue perfusion* |
7 (16%) |
103 (103–119) |
|||||||||
* For definitions see Box 1. |
|||||||||||
Received 12 March 2002, accepted 14 August 2002
- Craig J French1
- Rinaldo Bellomo2
- Simon R Finfer3
- Jeffery Lipman4
- Marianne Chapman5
- Neil W Boyce6
- and the ANZICS Clinical Trials Group*
- 1 Department of Intensive Care, Western Health, Melbourne, VIC.
- 2 Department of Intensive Care, Austin and Repatriation Medical Centre, Melbourne, VIC.
- 3 Department of Intensive Care, Royal North Shore Hospital, Sydney, NSW.
- 4 Department of Intensive Care, Royal Brisbane Hospital, Brisbane, QLD.
- 5 Department of Intensive Care, Royal Adelaide Hospital, Adelaide, SA.
- 6 Australian Red Cross Blood Service, Melbourne, VIC.
None identified.
- 1. Corwin HL, Parsonnet KC, Gettinger A. RBC transfusion in the ICU. Is there a reason? Chest 1995; 108: 767-771.
- 2. Hebert PC, Wells G, Tweeddale M, et al. Does transfusion practice affect mortality in critically ill patients? Transfusion Requirements in Critical Care (TRICC) Investigators and the Canadian Critical Care Trials Group. Am J Respir Crit Care Med 1997; 155: 1618-1623.
- 3. Corwin HL. Blood transfusion: first, do no harm! Chest 1999; 116: 1149-1150.
- 4. Hetzel JR. Managing the mismatch between blood supply and clinical demand. Med J Aust 1998; 169: 407-408.
- 5. Tobin SN, Campbell DA, Boyce NW. Durability of response to a targeted intervention to modify clinician transfusion practices in a major teaching hospital. Med J Aust 2001; 174: 445-448.
- 6. Rubin GL, Schofield WN, Dean MG, Shakeshaft AP. Appropriateness of red blood cell transfusions in major urban hospitals and effectiveness of an intervention. Med J Aust 2001; 175: 354-358.
- 7. Metz J, McGrath KM, Copperchini ML, et al. Appropriateness of transfusions of red cells, platelets and fresh frozen plasma. An audit in a tertiary care teaching hospital. Med J Aust 1995; 162: 572-573.
- 8. Vincent JL, Gattinoni L, Reinhart K, et al. Anaemia and blood transfusion in critical care (ABC) survey: organ function and mortality in transfused patients. Intensive Care Med 2001; 27 Suppl 2: S247.
- 9. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818-829.
- 10. National Health and Medical Research Council. Clinical practice guidelines: appropriate use of red blood cells. <http://www.health.gov.au/nhmrc/publications/_files/cp81.pdf> Canberra: NHMRC, 2001.
- 11. Access 97 [computer program]. Seattle, WA: Microsoft, 1997.
- 12. Analyse-it [computer program]. Version 1.63. Leeds, UK: Analyse-It Software Ltd, 2002.
- 13. Hebert PC, Wells G, Martin C, et al. A Canadian survey of transfusion practices in critically ill patients. Transfusion Requirements in Critical Care Investigators and the Canadian Critical Care Trials Group. Crit Care Med 1998; 26: 482-487.
- 14. Cane RD. Hemoglobin: how much is enough? Crit Care Med 1990; 18: 1046-1047.
- 15. Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999; 340: 409-417.
- 16. Bellomo R. Will less liberal red-cell transfusion (with a lower haemoglobin threshold) still reduce rates of death and organ failure? Med J Aust 2001; 175: 387.
- 17. Hebert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases. Crit Care Med 2001; 29: 227-234.
- 18. Wu WC, Rathore SS, Wang Y, et al. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med 2001; 345: 1230-1236.





Abstract
Objective: To determine the incidence and appropriateness of use of allogenic packed red blood cell (RBC) transfusion in Australian and New Zealand intensive care practice.
Setting: Intensive care units of 18 Australian and New Zealand hospitals: March 2001.
Design: Prospective, observational, multicentre study.
Methods: All admissions to participating intensive care units were screened and all patients who received a transfusion of RBC were enrolled. The indications for transfusion were recorded and compared with Australian National Health and Medical Research Council guidelines. Transfusions conforming to these guidelines were deemed appropriate.
Main outcome measures: RBC transfusion in intensive care and transfusion appropriateness.
Results: 1808 admissions to intensive care units were screened: 357 (19.8%) admissions (350 patients) received an RBC transfusion while in intensive care. Overall, 1464 RBC units were administered in intensive care on 576 transfusion days. The most common indications for transfusion were acute bleeding (60.1%; 880/1464) and diminished physiological reserve (28.9%; 423/1464). The rate of inappropriate transfusion was 3.0% (44/1464). Diminished physiological reserve with haemogloblin level ≥ 100 g/L was the indication in 50% (22/44) of inappropriate transfusions; no indication was provided for 31% (15/44).
Conclusion: The rate of inappropriate transfusion in Australian and New Zealand intensive care units in 2001 was remarkably low.