The known: Lung cancer screening with low‐dose computed tomography (LDCT) is associated with lower lung cancer‐related mortality among people at high risk of the disease.
The new: Incidental findings in 73% of Australian and Canadian participants in an LDCT lung cancer screening trial included findings requiring clinical attention in 10% of people. The reporting of incidental findings in clinical reports for treating physicians was inconsistent.
The implications: The new Australian lung cancer screening program should include standardised reporting of incidental findings, as they have consequences for the wellbeing of screening participants and the cost‐effectiveness of the screening program.
Lung cancer is the leading cause of cancer‐related death in Australia and overseas.1 Screening for lung cancer using low‐dose computed tomography (LDCT) reduces lung cancer‐related mortality in people at particular risk of lung cancer with an active tobacco smoking history.2,3,4 The Australian Department of Health and Aged Care plans to commence a national LDCT lung cancer screening program by mid‐2025.5
Incidental findings are a major consideration for lung cancer screening using LDCT. Unlike other cancer screening techniques, it can detect tissue changes other than lung cancer and pulmonary nodules. The largest relevant lung cancer screening randomised controlled trial to date, the United States National Lung Screening Trial, reported that significant incidental findings were identified in 33.8% of people in the LDCT group, most frequently emphysema (43% of participants) and coronary artery calcification (12%).6
Some incidental findings could facilitate earlier detection of clinically significant changes, such as coronary artery calcification and cardiovascular disease, and vertebral fractures and osteoporosis. However, LDCT findings of thyroid nodules,7 for example, could lead to unnecessary investigations and consequently increase the harms and costs of lung cancer screening. In Europe and the United States, guidelines include recommendations about which incidental findings are clinically relevant, and which are probably not.8,9,10
The prevalence of incidental findings in American6 and European lung cancer screening cohorts11 has been reported, but not for an Australian or Canadian lung cancer screening trial cohort. We therefore prospectively investigated the type and frequency of incidental findings in people at high risk of lung cancer who underwent LDCT screening in Australia and Canada.
Methods
The International Lung Screen Trial (ILST) is a multisite single arm LDCT lung cancer screening study; its primary objectives are to compare lung cancer screening participant selection criteria and to evaluate a nodule management protocol.12 The target sample size was 4000 participants from Australia (five sites, selected by geographic location and local expertise) and Canada (one site, selected for its available infrastructure and expertise);12 the overall target was subsequently increased after the study commenced because greater resources allowed the addition of ILST sites in Hong Kong, the United Kingdom, and Spain.13,14 The ILST was registered with the United States Clinical Trials Registry on 18 August 2016 (clinicaltrials.gov: NCT02871856); recruitment commenced in Australia and Canada on 25 August 2016 and concluded on 21 November 2020.
Participants for the ILST were recruited through social media, primary care providers, government mailouts, news and radio advertising, and personal referrals from friends and family.13 People were eligible to participate if they were aged 55–80 years, had an active smoking history, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and either an estimated six‐year lung cancer risk of 1.51% or more, based on the PLCOm2012 risk prediction model, or a smoking history of 30 pack‐years or more (US Preventive Task Force 2013 criterion).15 The PLCOm2012 is a logistic regression model that incorporates age, race or ethnic group, education level, body mass index, history of chronic obstructive pulmonary disease (COPD), family history of lung cancer, personal history of cancer, and smoking history to estimate the risk of lung cancer;16 the US Preventive Task Force 2013 criteria, in contrast, are based solely on age and smoking history.15 The PLCOm2012 has been validated in Australia and Canadian cohorts of people with active tobacco smoking histories.17
Baseline low‐dose computed tomography lung cancer screening
The prospective observational study reported in this article, nested within the ILST, included all Australian (Brisbane: Prince Charles Hospital; Melbourne: [I] Royal Melbourne Hospital and [II] Epworth Box Hill Hospital; Perth: Fiona Stanley Hospital; Sydney: St Vincent's Hospital) and Canadian (Vancouver General Hospital) ILST sites. The first baseline LDCT screens were undertaken in August 2016, and the final baseline LDCT screens in July 2021. We report our study in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.18
The primary aim of the study was to determine the prevalence of incidental findings in baseline LDCT screens in an Australian and Canadian lung cancer screening sample. The secondary aim was to describe the pattern of clinical reporting of LDCT lung cancer screening in Australia, based on a subset of ILST participants.
All participants underwent baseline LDCT screening for lung cancer; further interval scans and investigations for lung cancer were undertaken according to the trial protocol, using the PanCan nodule calculator score.12 LDCT (120 kV tube voltage, 40–50 mA tube current) was performed without intravenous contrast in the supine position during a single inspiratory breath hold. LDCT reporting was completed by experienced chest radiologists (at least three hundred computed tomography [CT] chest readings during the past three years) using a standardised research template.
Age, sex, smoking status, smoking history (in pack‐years), ethnic background, other medical conditions, medications, and spirometry findings were collected at baseline in the health questionnaire and assessment for all enrolled ILST participants.
Incidental findings
In addition to nodules and findings relevant to lung cancer, information on incidental findings unrelated to lung cancer was collected using a checklist. Incidental findings were categorised as present or absent for emphysema, interstitial lung abnormalities, airway abnormalities (mucous impaction, bronchial wall thickening, bronchiectasis, bronchiolectasis), and coronary artery calcification; other incidental findings — pleural, cardiovascular, gastrointestinal, endocrine, musculoskeletal, breast, and lymph node findings — were recorded as actionable (requiring clinical follow‐up) or non‐actionable. If the reporting radiologist did not select an option for an incidental finding in the research checklist, it was treated as missing information and the participant was excluded from the summary statistic for the finding. The total number of participants with complete information for each incidental finding included in the checklist was recorded. The research checklist was a data collection tool for the ILST and was not provided to participants or treating clinicians. ILST radiologists instead provided a clinical report for each participant's treating clinicians, which also specified lung cancer risk and recommended screening follow‐up. Classification and reporting of incidental findings were at the radiologists’ discretion, and there was no pre‐specified method or template for reporting incidental findings in LDCT clinical reports. A consensus guide to incidental findings definitions, including their categorisation, is included in the Supporting Information, table 1.
To assess the communication of incidental findings to clinicians, all incidental findings in Brisbane and Melbourne (I) baseline LDCT screening clinical reports were manually extracted by a clinician (Melbourne [I]: author AB; Brisbane: author HM) and classified as requiring action if included in the conclusion of the clinical report, or as not requiring action if included only in the body of the report or not at all. Retrospective review of baseline LDCT clinical reports was limited to these two sites for resource reasons.
Statistical analysis
Statistical analyses were performed in R 4.2.1 (R Foundation for Statistical Computing). We summarise parametric data as means with standard deviations (SDs) and non‐parametric data as medians with interquartile ranges (IQRs). The statistical significance of between‐group differences were assessed in Pearson χ2 and Fisher exact tests (categorical variables); P < 0.05 was deemed statistically significant.
Ethics approval
This study was approved by the Prince Charles Hospital human research ethics committee (HREC/16/QPCH/181); all relevant local governance approvals were obtained. All study participants provided written informed consent to participation in the study.
Results
A total of 4403 participants were enrolled and completed baseline LDCT screening at the six participating hospitals (Box 1). The mean age (64–65 years) and the proportions of participants who currently smoked (47–55%) were similar at all six sites; the proportion of female participants was larger in Sydney (52%) and Vancouver (51%) than at the other sites (39–44%). Median smoking history exceeded 40 pack‐years at all sites; it was slightly higher at Australian sites than in Vancouver, where median PLCOm2012 scores were also slightly higher. Most participants were classified as having European ethnic backgrounds (86–97% by site); the proportion of Indigenous participants was slightly higher in Brisbane (2.4%) and Vancouver (2.3%) than at the other sites (0–1.6%) (Box 2). No baseline medical conditions were reported by 2928 participants (67%) (Supporting Information, table 2).
Prevalence of incidental findings at baseline LDCT screening
At least one incidental finding was made during baseline LDCT screening of 3225 people (72.8%); findings in 454 people (10.3%) required clinical follow‐up or action, including 351 of 2099 Australian (16.7%) and 103 of 2304 Canadian participants (4.5%). Data completeness varied by research checklist incidental finding type (Box 3).
Of the 2378 participants with incidental findings of radiological emphysema (54.0%), 1563 (65.7%) reported no history of obstructive airways disease. Ninety‐one of 362 participants with incidental findings of moderate to severe emphysema (25.1%) reported a history of emphysema, including seven of nineteen with incidental findings of severe emphysema. Two of 541 participants with incidental findings of interstitial lung abnormalities reported a history of pulmonary fibrosis.
Among the 3022 participants with incidental findings of coronary artery calcification (69.0%), 94 of 1649 with mild calcification (5.7%), 96 of 759 with moderate calcification (12.6%), and 159 of 614 (25.9%) with severe coronary artery calcification reported histories of coronary artery disease.
Eighteen of 105 people with incidental endocrine findings requiring action (17%) and 30 of 176 with incidental endocrine findings that did not require follow‐up (17%) reported histories of thyroid disorder. Histories of osteoporosis or osteopenia were reported by 24 of 154 people with incidental vertebral findings that required action (15.6%) and 50 of 271 people with incidental vertebral findings that did not require follow‐up (18.5%).
Clinical reporting of baseline LDCT screening incidental findings
As the baseline reports for all site participants from the Brisbane and Melbourne (I) sites were included in the retrospective review of baseline LDCT clinical reports, there were no missing data (Box 4; Supporting Information, table 3).
Respiratory system findings
Emphysema was mentioned in LDCT clinical reports as a finding requiring action for 69 of 570 participants at the two sites with incidental findings of radiological emphysema (12%); twelve of these participants (17%) reported a history of COPD. Increasing severity of emphysema was significantly associated with being reported in the conclusion of Brisbane clinical reports (P < 0.001) (Supporting Information, table 4). For Melbourne (I), emphysema requiring action was reported in five of 408 clinical LDCT reports; the one incidental finding of severe emphysema was not reported as requiring action. Forty‐eight of 618 incidental emphysema findings (7.8%) were not mentioned in LDCT clinical reports.
None of the 45 participants with actionable incidental findings of interstitial lung abnormalities had known pulmonary fibrosis at enrolment; five reported prior pneumonia.
Incidental findings of bronchiectasis were not mentioned in the clinical reports for 171 of 296 people.
Incidental findings of coronary artery calcification
For 32 of 705 participants with incidental findings of coronary artery calcification (4.7%), this finding was not mentioned in their LDCT clinical reports, including fourteen with findings of moderate to severe coronary artery calcification; incidental findings of severe coronary artery calcification were mentioned in the report conclusion for 33 of 71 people (46%). Larger proportions of findings of moderate (93 of 197, 47.2%) or severe coronary artery calcification (33 of 71, 46.5%) were reported in report conclusions than of mild calcification (85 of 413, 20.6%) (Supporting Information, table 5).
Other system findings
Incidental findings of thyroid abnormalities were reported in the LDCT clinical report conclusion for all 29 people with incidental findings deemed actionable, nineteen of whom did not report histories of thyroid disease.
Ninety‐four of 113 participants with incidental liver findings did not have histories of liver disease, including three of 35 participants with incidental liver findings requiring action.
Five of 60 participants with incidental renal abnormality findings (8%) reported histories of renal disease.
Incidental findings of vertebral fractures 43 of 155 people (27.7%) were not mentioned in LDCT reports.
Discussion
We report the first investigation of incidental findings in LDCT lung cancer screening of a large cohort of participants in Australia and Canada. Incidental findings were frequent (72.8% of participants); incidental findings requiring clinical follow‐up were made for 16.7% of participants in Australia. The most frequent findings were coronary artery calcification (69.0% of participants) and emphysema (54.0%). which is unsurprising for people at high risk of respiratory disease with substantial tobacco smoking histories. Coronary artery calcification was also the most frequent incidental finding in a London cohort of people at high risk of lung cancer screened using LDCT (64.2% of 11 115 participants).19 Clinically significant incidental findings were reported for 33.8% of 26 455 LDCT screening participants in the United States National Lung Screening Trial, most frequently emphysema (43.0%).6
The marked differences between the Australian and Canadian sites in the prevalence of incidental findings could be related to differences in population characteristics or in radiology reporting practices. Study participants were selected because they were at high risk of lung cancer, and large proportions of participants at all sites currently smoked, but the median smoking history at Australian sites was greater than for Vancouver. All Canadian LDCT screening reports were prepared by a single experienced chest radiologist, the Australian reports by several experienced chest radiologists. Even within Australia, however, the reporting of certain incidental findings as requiring action (potentially clinically significant) in clinical reports for treating clinicians differed between the two sites examined; for example, coronary artery calcification requiring follow‐up was reported in a larger proportion of LDCT clinical reports from Brisbane (29.1%) than of those from Melbourne (I) (10.6%).
Our study was conducted under real world conditions, and our findings illustrate the potential for variation in recommendations and care when there is no clear and consistent guidance about reporting. The Yale Lung Screening and Nodule Program has proposed structured LDCT screening reports, incorporating not only a description of potentially significant incidental findings (S classification of the Lung Imaging Reporting and Data System, Lung–RADS), but also, when relevant, a findings summary and guideline‐based management recommendations in the report conclusion.10 Similar recommendations proposed in Europe9 recognise the possibility of inappropriate investigation and management of incidental findings. It is important for lung cancer screening programs that radiologists uniformly characterise and describe incidental findings, and that they provide consistent recommendations regarding the need for further assessment in a structured format. Not including some clinically insignificant incidental findings in the LDCT report could reduce the risk of unnecessary investigations. It is also critical that clinicians and screening participants are aware that incidental findings are possible during LDCT lung cancer screening.
In our study, the level of missing research checklist data ranged from two participants (0.05%) with no recorded emphysema outcome to 129 participants (2.9%) without recorded interstitial lung abnormality status. The reporting of incidental findings was inconsistent in an observational study of 37 908 people in 43 United States facilities even after the addition of the S category to Lung–RADS for potentially clinically significant incidental findings; it was used in 0.1% to 37.4% of initial LDCT reports, depending on the site.20 Differences between narrative lung cancer screening LDCT reports and synoptic reporting in Canada have been described, with at least one omission noted in 70% of narrative reports.21 This suggests that the quality of LDCT lung cancer screening reports should be reviewed and benchmarked across screening sites to ensure that programs provide optimal care.
Most participants with incidental findings in our study did not report relevant clinical histories; for example, fewer than 1% of people with incidental interstitial lung abnormality findings reported pulmonary fibrosis. At a single site in the United States National Lung Screening Trial, incidental interstitial lung abnormality findings were recorded for 9.7% of participants (and was associated with current active tobacco smoking); equivocal lung parenchymal changes were incidental findings in a further 11.5%.22 The European joint statement on the management of incidental findings recommended reporting all interstitial lung abnormality findings and further surveillance, or referring the person to a specialist if more than 5% of a whole lung or zone was involved.9 The Fleischner Society similarly recommends clinical assessment before deciding on further management.23 The workload for primary care providers is increased if clear guidance and streamlined processes are not available. In the Manchester Lung Health Check pilot screening study, the initial protocol for notifying primary care providers about additional findings and recommended management was modified to reduce the burden on primary care, and the lung cancer screening team assumed responsibility for any specialist referrals.11
Although inappropriate investigation of incidental findings could adversely affect screening participants and the cost‐effectiveness of LDCT lung cancer screening, clinically significant incidental findings could also improve health care. We have previously discussed, for example, incorporating incidental coronary artery calcification findings into cardiovascular disease risk assessment and care optimisation.24 The second most frequent incidental finding in our study was emphysema, found in more than half of the participants, if mostly trivial or mild in severity. Most people with incidental findings of moderate to severe emphysema reported no history of the condition, and lung cancer screening could be an opportunity for earlier detection and intervention in people who have not reported COPD symptoms.25 The European statement on the management of incidental findings recommends smoking cessation for all people with incidental emphysema findings, and clinical assessment of those in whom it is moderate to severe.9 The American College of Radiology, however, recommends primary care assessment, regardless of severity, and consideration of referral to a respiratory medicine specialist.8 Local guidance that takes health care infrastructure and resources into account is important. A recent survey of primary care providers in the United States found that adherence to guidelines for the management of incidental findings was higher when LDCT reports used a standardised category S template (83.3%) than when they did not (51.7%).26 This finding was consistent with another that including radiologist recommendations in the LDCT report body (odds ratio [OR], 4.67; 95% confidence interval [CI], 2.23–9.76) and conclusion (OR, 2.58; 95% CI, 1.28–5.18) each increased the likelihood of the incidental finding being investigated further.27
Limitations
The ILST did not provide strict guidance regarding which incidental findings did or did not require clinical follow‐up, and decisions about reporting them were at the discretion of the radiologist, reflecting usual practice. This situation probably contributed to variations in reporting by the participating radiologists. A large volume of information was collected with the LDCT screening research template, including several fields for incidental findings. Missing data may reflect the effort required to complete a comprehensive LDCT lung cancer screening report, which includes incidental findings as well as lung cancer risk; this factor will be important when structuring LDCT screening reports for the Australian lung cancer screening program. There were some minor discrepancies between incidental findings recorded in the research template and those recorded in narrative clinical reports; they may reflect errors at the time of reporting or differences in recording findings for research and clinical purposes. Further, the ILST commenced before most overseas recommendations for managing incidental findings had been published. The marked differences between the Australian and Canadian sites in the proportions of incidental findings deemed to require clinical follow‐up could be related to cultural or population differences, or to a single radiologist reporting all findings for the one Canadian site. Resource limitations meant that incidental finding reporting could only be reviewed for LDCT reports from two Australian sites. The results of multiple independent statistical tests should be interpreted with caution, given the increasing risk of type 1 errors with multiple comparisons.
As the large majority of ILST participants had European ethnic backgrounds, our findings must be generalised to people with other ethnic backgrounds with caution. The participating sites may not necessarily be representative of their respective countries. As Aboriginal and Torres Strait Islander Australians are at greater risk of lung cancer and lung cancer‐related mortality than other Australians,28 major health benefits could be achieved by a successful lung cancer screening program for Indigenous Australians.29 However, more information about incidental finding rates in Aboriginal and Torres Strait Islander people is needed; the Australian Lung Screen Trial, which recently commenced recruitment, will investigate lung cancer screening of Indigenous people living in rural and remote communities.30
Information about other medical conditions and medications were based on participant responses to baseline questionnaires. Participants may have not reported known conditions or been unaware of some conditions, which could mitigate the clinical significance of some incidental findings. We did not assess subsequent investigations or the management of incidental findings to evaluate their impact on lung cancer screening participants, including investigation harms and costs. Five‐year follow‐up outcomes, including subsequent investigations and costs of screening for Australian ILST participants, will be reported as they become available.
Conclusion
Incidental findings were recorded during LDCT lung cancer screening for 72.8% of Australian and Canadian participants aged 55–80 years at high risk of lung cancer, including clinically significant findings in 10.3% of participants. Our findings will be a useful reference for lung cancer screening programs in the two countries. When lung cancer screening commences in Australia, the judicious reporting of incidental findings could increase the benefits and reduce the harms of the program. All screening sites should consider structured reporting of incidental findings and provide clear guidelines regarding their management according to local standards of care. LDCT lung cancer screening reduces lung cancer‐related mortality among people at particular risk of the disease, but the impact of incidental findings on outcomes should be investigated, including complications of investigations, participant distress, and program cost‐effectiveness.
Box 1 – Participant selection for International Lung Screen Trial (ILST) substudy of incidental findings during low‐dose computed tomography lung cancer screening in Australia (five sites) and Canada (one site), 2016–2021
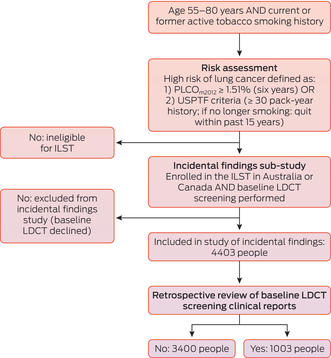
LDCT = low‐dose computed tomography; USPTF = United States Preventive Task Force.
Box 2 – Baseline characteristics of participants in the International Lung Screen Trial substudy of incidental findings during low‐dose computed tomography lung cancer screening in Australia (five sites) and Canada (one site), 2016–2021
Characteristic |
Brisbane |
Melbourne (I) |
Melbourne (II) |
Perth |
Sydney |
Vancouver | |||||||||
Number of participants |
595 |
408 |
127 |
591 |
378 |
2304 |
|||||||||
Age (years), mean (SD) |
64 (6.4) |
64 (6.1) |
65 (6.5) |
65 (6.5) |
65 (6.1) |
64 (6.4) |
|||||||||
Sex |
|||||||||||||||
Women |
235 (39%) |
167 (41%) |
56 (44%) |
242 (41%) |
196 (52%) |
1179 (51%) |
|||||||||
Men |
360 (61%) |
241 (59%) |
71 (56%) |
349 (59%) |
182 (48%) |
1125 (49%) |
|||||||||
Currently smokes tobacco |
281 (47%) |
225 (55%) |
69 (54%) |
287 (49%) |
193 (51%) |
1095 (48%) |
|||||||||
Smoking history (pack‐years), median (IQR) |
44 (35–58) |
46 (36–57) |
43 (34–54) |
43 (34–54) |
42 (34–55) |
40 (33–49) |
|||||||||
PLCOm2012 score, median (IQR) |
2.9 (1.9–4.8) |
3.1 (1.9–5.1) |
2.7 (1.7–4.9) |
2.7 (1.7–4.9) |
3.1 (2.0–5.3) |
2.6 (1.7–4.3) |
|||||||||
Ethnic background |
|||||||||||||||
African |
2 (0.3%) |
1 (0.5%) |
0 |
1 (0.2%) |
1 (0.3%) |
27 (1.2%) |
|||||||||
Asian |
2 (0.3%) |
8 (2.0%) |
4 (3.1%) |
8 (1.4%) |
2 (0.5%) |
225 (9.8%) |
|||||||||
European |
576 (97%) |
391 (96%) |
113 (89%) |
570 (96%) |
365 (96%) |
1984 (86%) |
|||||||||
Indigenous |
14 (2.4%) |
3 (0.7%) |
0 |
3 (0.5%) |
6 (1.6%) |
53 (2.3%) |
|||||||||
Other |
1 (0.2%) |
5 (1.2%) |
10 (7.9%) |
9 (1.5%) |
4 (1.1%) |
15 (0.7%) |
|||||||||
Education (highest level) |
|||||||||||||||
Year 8 or lower |
221 (37%) |
130 (32%) |
33 (26%) |
217 (37%) |
108 (29%) |
248 (11%) |
|||||||||
Year 8 to 11 |
86 (14%) |
80 (20%) |
26 (20%) |
118 (20%) |
67 (18%) |
564 (24%) |
|||||||||
High school graduate |
108 (18%) |
57 (14%) |
20 (16%) |
109 (18%) |
84 (22%) |
316 (14%) |
|||||||||
Vocational certificate |
77 (13%) |
43 (11%) |
8 (6%) |
58 (10%) |
20 (5.3%) |
569 (25%) |
|||||||||
Some university/college |
66 (11%) |
54 (13%) |
26 (20%) |
67 (11%) |
61 (16%) |
403 (17%) |
|||||||||
University graduate |
37 (6%) |
42 (10%) |
14 (11%) |
22 (4%) |
38 (10%) |
204 (9%) |
|||||||||
Unknown |
0 |
2 (0.5%) |
0 |
0 |
0 |
0 |
|||||||||
Spirometry |
|||||||||||||||
FEV1 (% predicted), median (IQR) |
87% (85–88%) |
94% (92–95%) |
90% (86–93%) |
90% (88–92%) |
87% (85–89%) |
88% (87–89%) |
|||||||||
Forced expiratory ratio, median (IQR) |
66% (66–67%) |
70% (69–71%) |
71% (70–73%) |
69% (68–69%) |
73% (72–74%) |
70% (70–71%) |
|||||||||
FEV1 = forced expiratory volume in one second; IQR = interquartile range; SD = standard deviation. | |||||||||||||||
Box 3 – Incidental findings in the International Lung Screen Trial substudy of incidental findings during low‐dose computed tomography lung cancer screening in Australia (five sites) and Canada (one site), 2016–2021, by country
Finding type |
Australia |
Canada |
P* |
Missing data (Australia/Canada) | |||||||||||
Total number of people |
2099 |
2304 |
|||||||||||||
Pleura |
< 0.001 |
6/0 |
|||||||||||||
Actionable |
22/2093 (1.1%) |
5/2304 (0.2%) |
|||||||||||||
Non‐actionable |
166/2093 (7.9%) |
55/2304 (2.4%) |
|||||||||||||
Normal |
1905/2093 (91.0%) |
2244/2304 (97.4%) |
|||||||||||||
Interstitial lung abnormality |
< 0.001 |
129/0 |
|||||||||||||
Actionable |
101/1970 (6.3%) |
2/2304 (0.1%) |
|||||||||||||
Non‐actionable |
18/1970 (1.1%) |
66/2304 (2.9%) |
|||||||||||||
Normal |
1497/1970 (92.6%) |
2236/2304 (97.0%) |
|||||||||||||
Airways |
< 0.001 |
20/30 |
|||||||||||||
Abnormal |
683/2079 (32.9%) |
534/2274 (23.5%) |
|||||||||||||
Normal |
1396/2079 (67.1%) |
1740/2274 (76.5%) |
|||||||||||||
Emphysema |
< 0.001 |
2/0 |
|||||||||||||
Present |
1186/2097 (57.0%) |
1192/2304 (51.7%) |
|||||||||||||
Absent |
911/2097 (43.4%) |
1112/2304 (48.3%) |
|||||||||||||
Emphysema (by grade) |
— |
— |
|||||||||||||
Trivial (< 5%) |
407/1186 (34.3%) |
834/1192 (70.0%) |
|||||||||||||
Mild (5–25%) |
518/1186 (43.7%) |
244/1192 (20.5%) |
|||||||||||||
Moderate (> 25% to 50%) |
186/1186 (15.7%) |
80/1192 (6.7%) |
|||||||||||||
Marked (> 50% to 75%) |
52/1186 (4.4%) |
25/1192 (2.1%) |
|||||||||||||
Severe (> 75%) |
12/1186 (1.0%) |
7/1192 (0.6%) |
|||||||||||||
Not recorded |
11/1186 |
2/1192 |
|||||||||||||
Coronary artery calcification |
< 0.001 |
19/4 |
|||||||||||||
Present |
1396/2080 (67.1%) |
1626/2300 (70.7%) |
|||||||||||||
Absent |
684/2080 (32.9%) |
674/2300 (29.3%) |
|||||||||||||
Coronary artery calcification (by grade) |
— |
— |
|||||||||||||
Mild |
803/2080 (38.6%) |
846/2300 (36.8%) |
|||||||||||||
Moderate |
425/2080 (20.4%) |
334/2300 (14.5%) |
|||||||||||||
Severe |
168/2080 (8.1%) |
446/2300 (19.4%) |
|||||||||||||
Cardiovascular (excluding coronary artery calcification) |
< 0.001 |
6/2 |
|||||||||||||
Actionable |
140/2093 (6.7%) |
19/2302 (0.8%) |
|||||||||||||
Non‐actionable |
308/2093 (14.7%) |
204/2302 (8.9%) |
|||||||||||||
Normal |
1645/2093 (78.6%) |
2079/2302 (90.3%) |
|||||||||||||
Gastrointestinal |
< 0.001 |
4/2 |
|||||||||||||
Actionable |
81/2095 (3.9%) |
24/2302 (1.0%) |
|||||||||||||
Non‐actionable |
376/2095 (17.9%) |
629/2302 (27.3%) |
|||||||||||||
Normal |
1638/2095 (78.2%) |
1649/2302 (71.6%) |
|||||||||||||
Endocrine |
< 0.001 |
9/1 |
|||||||||||||
Actionable |
73/2090 (3.5%) |
32/2303 (1.4%) |
|||||||||||||
Non‐actionable |
79/2090 (3.8%) |
97/2303 (4.2%) |
|||||||||||||
Normal |
1938/2090 (97.7%) |
2174/2303 (94.4%) |
|||||||||||||
Musculoskeletal |
< 0.001 |
7/1 |
|||||||||||||
Actionable |
32/2092 (1.5%) |
6/2303 (0.3%) |
|||||||||||||
Non‐actionable |
425/2092 (20.3%) |
180/2303 (7.8%) |
|||||||||||||
Normal |
1635/2092 (78.2%) |
2117/2303 (91.9%) |
|||||||||||||
Vertebral |
< 0.001 |
125/3 |
|||||||||||||
Actionable |
150/1974 (7.6%) |
4/2301 (0.2%) |
|||||||||||||
Non‐actionable |
23/1974 (1.2%) |
248/2301 (10.8%) |
|||||||||||||
Normal |
1801/1974 (91.2%) |
2049/2301 (89.0%) |
|||||||||||||
Breast |
0.15 |
6/2 |
|||||||||||||
Actionable |
29/2093 (1.4%) |
14/2302 (0.6%) |
|||||||||||||
Non‐actionable |
44/2093 (2.1%) |
90/2302 (3.9%) |
|||||||||||||
Normal |
2020/2093 (96.5%) |
2198/2302 (95.5%) |
|||||||||||||
Lymph nodes (axillary and abdominal) |
< 0.001 |
5/2 |
|||||||||||||
Actionable |
35/2094 (1.7%) |
8/2302 (0.3%) |
|||||||||||||
Non‐actionable |
58/2094 (2.8%) |
29/2302 (1.3%) |
|||||||||||||
Normal |
2001/2094 (95.6%) |
2265/2302 (98.4%) |
|||||||||||||
Other |
< 0.001 |
31/2 |
|||||||||||||
Actionable |
76/2068 (3.7%) |
43/2302 (1.9%) |
|||||||||||||
Non‐actionable |
161/2068 (7.8%) |
780/2302 (33.9%) |
|||||||||||||
Normal |
1831/2068 (88.5%) |
1479/2302 (64.2%) |
|||||||||||||
* Fisher exact test. | |||||||||||||||
Box 4 – Incidental findings reported in screening clinical reports for participants screened at the Brisbane or Melbourne (I) sites*
Finding type |
Brisbane |
Melbourne (I) |
P † | ||||||||||||
Total number of people |
595 |
408 |
|||||||||||||
Emphysema |
< 0.001 |
||||||||||||||
Actionable |
64 (10.8%) |
5 (1.2%) |
|||||||||||||
Non‐actionable |
268 (45.0%) |
233 (57.1%) |
|||||||||||||
Not reported‡ |
21 (3.5%) |
27 (6.6%) |
|||||||||||||
Pulmonary fibrosis |
0.23 |
||||||||||||||
Actionable |
10 (1.7%) |
2 (0.5%) |
|||||||||||||
Non‐actionable |
25 (4.2%) |
17 (4.2%) |
|||||||||||||
Interstitial lung abnormality |
0.21 |
||||||||||||||
Actionable |
24 (4.0%) |
21 (5.1%) |
|||||||||||||
Non‐actionable |
7 (1.2%) |
10 (2.5%) |
|||||||||||||
Bronchiectasis |
0.006 |
||||||||||||||
Actionable |
23 (3.9%) |
10 (2.5%) |
|||||||||||||
Non‐actionable |
41 (6.9%) |
51 (12.5%) |
|||||||||||||
Not reported‡ |
99 (16.7%) |
72 (17.6%) |
|||||||||||||
Bronchial wall thickening |
0.002 |
||||||||||||||
Actionable |
21 (3.5%) |
2 (0.5%) |
|||||||||||||
Non‐actionable |
51 (8.6%) |
49 (12.0%) |
|||||||||||||
Pleura |
< 0.001 |
||||||||||||||
Actionable |
25 (4.2%) |
10 (2.5%) |
|||||||||||||
Non‐actionable |
51 (8.6%) |
12 (2.9%) |
|||||||||||||
Coronary artery calcification |
< 0.001 |
||||||||||||||
Actionable |
173 (29.1%) |
43 (10.6%) |
|||||||||||||
Non‐actionable |
245 (41.2%) |
212 (52.1%) |
|||||||||||||
Not reported‡ |
8 (1.3%) |
24 (5.9%) |
|||||||||||||
Valves |
< 0.001 |
||||||||||||||
Actionable |
7 (1.2%) |
0 |
|||||||||||||
Non‐actionable |
42 (7.1%) |
3 (0.7%) |
|||||||||||||
Pericardium |
0.005 |
||||||||||||||
Actionable |
0 |
7 (1.7%) |
|||||||||||||
Non‐actionable |
0 |
0 |
|||||||||||||
Biliary |
0.005 |
||||||||||||||
Actionable |
3 (0.5%) |
4 (1.0%) |
|||||||||||||
Non‐actionable |
34 (5.7%) |
7 (1.7%) |
|||||||||||||
Liver |
0.011 |
||||||||||||||
Actionable |
20 (3.4%) |
15 (3.7%) |
|||||||||||||
Non‐actionable |
34 (5.7%) |
44 (10.8%) |
|||||||||||||
Pancreas |
0.07 |
||||||||||||||
Actionable |
1 (0.2%) |
5 (1.2%) |
|||||||||||||
Non‐actionable |
2 (0.3%) |
3 (0.7%) |
|||||||||||||
Spleen |
0.82 |
||||||||||||||
Actionable |
0 |
0 |
|||||||||||||
Non‐actionable |
8 (1.3%) |
4 (1.0%) |
|||||||||||||
Renal |
|||||||||||||||
Actionable |
5 (0.8%) |
16 (3.9%) |
0.002 |
||||||||||||
Non‐actionable |
20 (3.4%) |
19 (4.7%) |
|||||||||||||
Adrenal |
0.008 |
||||||||||||||
Actionable |
4 (0.7%) |
5 (1.2%) |
|||||||||||||
Non‐actionable |
1 (0.2%) |
8 (2.0%) |
|||||||||||||
Thyroid |
< 0.001 |
||||||||||||||
Actionable |
9 (1.5%) |
20 (4.9%) |
|||||||||||||
Non‐actionable |
8 (1.3%) |
20 (4.9%) |
|||||||||||||
Not reported‡ |
6 (1.0%) |
10 (2.5%) |
|||||||||||||
Diaphragm |
0.36 |
||||||||||||||
Actionable |
3 (0.5%) |
0 |
|||||||||||||
Non‐actionable |
12 (2.0%) |
8 (2.0%) |
|||||||||||||
Osteopenia |
< 0.001 |
||||||||||||||
Actionable |
7 (1.2%) |
1 (0.2%) |
|||||||||||||
Non‐actionable |
62 (10.4%) |
7 (1.7%) |
|||||||||||||
Vertebral fractures |
0.27 |
||||||||||||||
Actionable |
16 (2.7%) |
5 (1.2%) |
|||||||||||||
Non‐actionable |
55 (9.2%) |
36 (8.8%) |
|||||||||||||
Not reported‡ |
33 (5.5%) |
10 (2.5%) |
|||||||||||||
Breast |
0.08 |
||||||||||||||
Actionable |
13 (2.2%) |
2 (0.5%) |
|||||||||||||
Non‐actionable |
11 (1.8%) |
6 (1.5%) |
|||||||||||||
Lymph nodes: mediastinal/hilar |
0.022 |
||||||||||||||
Actionable |
15 (2.5%) |
7 (1.7%) |
|||||||||||||
Non‐actionable |
19 (3.2%) |
9 (2.2%) |
|||||||||||||
* As participants without the incidental findings are not included in this table, there were no missing data. † Fisher exact test, for actionable and non‐actionable findings in clinical reports. ‡ Incidental findings in research checklist not mentioned in clinical reports. | |||||||||||||||
Received 27 May 2024, accepted 21 October 2024
- Asha Bonney1,2
- Diane M Pascoe1,2
- Mark W McCusker1,2
- Daniel Steinfort1,2
- Henry Marshall3,4
- Annette McWilliams5,6
- Fraser J Brims7,8
- Emily Stone9,10
- Paul Fogarty11
- Jeremy D Silver12
- Brad Milner9
- Elizabeth Silverstone9
- Eugene Hsu9
- Duy Nguyen9
- Christopher Rofe9
- Cameron White9
- XinXin Hu9
- John Mayo13
- Renelle Myers14,15
- Kwun M Fong16
- Renee Manser1,2
- Stephen Lam14
- 1 Royal Melbourne Hospital, Melbourne, VIC
- 2 The University of Melbourne, Melbourne, VIC
- 3 The Prince Charles Hospital, Brisbane, QLD
- 4 Thoracic Research Centre, the University of Queensland, Brisbane, QLD
- 5 Fiona Stanley Hospital, Perth, WA
- 6 The University of Western Australia, Perth, WA.
- 7 Sir Charles Gairdner Hospital, Perth, WA
- 8 Curtin Medical School, Curtin University, Perth, WA
- 9 St Vincent's Hospital, Sydney, NSW
- 10 St Vincent's Clinical School, University of New South Wales, Sydney, NSW
- 11 Epworth Eastern Hospital, Melbourne, VIC
- 12 Statistical Consulting Centre, the University of Melbourne, Melbourne, VIC
- 13 Vancouver General Hospital, Vancouver, Canada
- 14 The University of British Columbia, Vancouver, Canada
- 15 BC Cancer, Vancouver, Canada
- 16 UQ Thoracic Research Centre, the Prince Charles Hospital, Brisbane, QLD
Open access:
Open access publishing facilitated by the University of Melbourne, as part of the Wiley – the University of Melbourne agreement via the Council of Australian University Librarians.
Data Sharing:
The study data can be accessed by contacting the corresponding author.
This study was supported by a National Health and Medical Research Council (NHMRC) project grant (1099154), an NHMRC postgraduate scholarship (Asha Bonney: APP2005195), a Royal Melbourne Hospital Emerging Research Leader Grant (Asha Bonney: GIA0462021) and a Medical Research Future Fund practitioner fellowship (Kwun M Fong: 1154446). The funding bodies had no role in the study design, interpretation of data or decision to submit the manuscript.
No relevant disclosures.
- 1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024; 74: 229‐263.
- 2. Bonney A, Malouf R, Marchal C, et al. Impact of low‐dose computed tomography (LDCT) screening on lung cancer‐related mortality. Cochrane Database Syst Rev 2022; 8: CD013829.
- 3. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung‐cancer mortality with volume CT screening in a randomized trial. N Engl J Med 2020; 382: 503‐513.
- 4. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med 2011; 365: 395‐409.
- 5. Australian Department of Health and Aged Care. National lung cancer screening program. Updated 13 Jan 2025. (viewed Jan 2025).
- 6. Gareen IF, Gutman R, Sicks J, et al. Significant incidental findings in the National Lung Screening Trial. JAMA Intern Med 2023; 183: 677‐684.
- 7. Jensen CB, Saucke MC, Francis DO, et al. From overdiagnosis to overtreatment of low‐risk thyroid cancer: a thematic analysis of attitudes and beliefs of endocrinologists, surgeons, and patients. Thyroid 2020; 30: 696‐703.
- 8. Dyer DS, White C, Conley Thomson C, et al. A quick reference guide for incidental findings on lung cancer screening CT examinations. J Am Coll Radiol 2023; 20: 162‐172.
- 9. O'Dowd EL, Tietzova I, Bartlett E, et al. ERS/ESTS/ESTRO/ESR/ESTI/EFOMP statement on management of incidental findings from low dose CT screening for lung cancer. Eur Respir J 2023; 62: 2300533.
- 10. Tanoue LT, Sather P, Cortopassi I, et al. Standardizing the reporting of incidental, non‐lung cancer (category S) findings identified on lung cancer screening low‐dose CT imaging. Chest 2022; 161: 1697‐1706.
- 11. Bradley P, Bola BM, Balata H, et al. Incidental findings in low dose CT lung cancer screening of high‐risk smokers: results from the Manchester lung health check pilot. Lung Cancer 2022; 173: 1‐4.
- 12. Lim KP, Marshall H, Tammemägi M, et al; ILST (International Lung Screening Trial) Investigator Consortium. Protocol and rationale for the International Lung Screening Trial. Ann Am Thorac Soc 2020; 17: 503‐512.
- 13. Tammemägi MC, Ruparel M, Tremblay A, et al. USPSTF2013 versus PLCOm2012 lung cancer screening eligibility criteria (International Lung Screening Trial): interim analysis of a prospective cohort study. Lancet Oncol 2022; 23: 138‐148.
- 14. Rosell Gratacos A, Baeza S, López‐Seguí F, et al. Implementation of the International Lung Screen Trial (ILST) in Catalonia: a cost analysis study [abstract: European Respiratory Society international congress, Barcelona, 4–6 Sept 2022]. Eur Respir J 2022; 60 (Suppl 66): 3070.
- 15. Moyer VA; US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2014; 160: 330‐338.
- 16. Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung‐cancer screening. N Engl J Med 2013; 368: 728‐736.
- 17. Weber M, Yap S, Goldsbury D, et al. Identifying high risk individuals for targeted lung cancer screening: independent validation of the PLCOm2012 risk prediction tool. Int J Cancer 2017; 141: 242‐253.
- 18. STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453‐1457.
- 19. Tisi S, Creamer AW, Dickson J, et al; SUMMIT Consortium. Prevalence and clinical characteristics of non‐malignant CT detected incidental findings in the SUMMIT lung cancer screening cohort. BMJ Open Respir Res 2023; 10: e001664.
- 20. Kearney LE, Butler C, Nunez ER, et al. Highly variable reporting of incidental findings in a national cohort of US veterans screened for lung cancer. Clin Imaging 2023; 100: 21‐23.
- 21. Tremblay A, Ezer N, Burrowes P, et al. Development and application of an electronic synoptic report for reporting and management of low‐dose computed tomography lung cancer screening examination. BMC Med Imaging 2022; 22: 111.
- 22. Jin GY, Lynch D, Chawla A, et al. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology 2013; 268: 563‐571.
- 23. Hatabu H, Hunninghake GM, Richeldi L, et al. Interstitial lung abnormalities detected incidentally on CT: a position paper from the Fleischner Society. Lancet Respir Med 2020; 8: 726‐737.
- 24. Bonney A, Chua M, McCusker MW, et al. Coronary artery calcification detected on low‐dose computed tomography in high‐risk participants of an Australian lung cancer screening program: a prospective observational study. Respirology 2025; 30: 62‐69.
- 25. Toelle BG, Xuan W, Bird TE, et al. Respiratory symptoms and illness in older Australians: the burden of obstructive lung disease (BOLD) study. Med J Aust 2013; 198: 144‐148. https://www.mja.com.au/journal/2013/198/3/respiratory‐symptoms‐and‐illness‐older‐australians‐burden‐obstructive‐lung
- 26. Kunitomo Y, Sather P, Killam J, et al. Impact of structured reporting for lung cancer screening low‐dose CT scan incidental findings on physician management. Chest 2024; 166: 896‐898.
- 27. Melzer AC, Atoma B, Fabbrini AE, et al. Variation in reporting of incidental findings on initial lung cancer screening and associations with clinician assessment. J Am Coll Radiol 2024; 21: 118‐127.
- 28. Australian Institute of Health and Welfare; Cancer Australia. Cancer in Aboriginal and Torres Strait Islander peoples of Australia: an overview (cat. no. CAN 75). 3 Oct 2013. https://www.aihw.gov.au/reports/cancer/cancer‐in‐indigenous‐australians‐overview/summary (viewed Mar 2025).
- 29. Brown A, Garvey G, Rankin NM, et al. Lung cancer screening for Aboriginal and Torres Strait Islander people: an opportunity to address health inequities. Med J Aust 2023; 219: 398‐401. https://www.mja.com.au/journal/2023/219/9/lung‐cancer‐screening‐aboriginal‐and‐torres‐strait‐islander‐peoples‐opportunity
- 30. Fong K. Australian lung screen trial for Aboriginal and Torres Strait Islander peoples. Australian New Zealand Clinical Trials Registry; Updated 8 May 2024. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12624000586516 (viewed Oct 2024).






Abstract
Objectives: To investigate the type and frequency of incidental findings in people at high risk of lung cancer who undergo baseline low‐dose computed tomography (LDCT) lung cancer screening in Australia and Canada.
Study design: Prospective observational study; sub‐study of the single‐arm International Lung Screen Trial (ILST) lung cancer screening study.
Setting, participants: Australian and Canadian people enrolled in the ILST, 25 August 2016 – 21 November 2020; inclusion criteria: aged 50–80 years, active smoking history, and high risk of lung cancer (estimated six‐year lung cancer risk of 1.51% or more, based on the PLCOm2012 risk prediction model; or a smoking history of 30 pack‐years or more). Initial LDCT screening was undertaken at one of five participating hospitals in Australia and one in Canada.
Main outcome measures: Prevalence of incidental findings during baseline LDCT lung cancer screening (using a research checklist), by country, classified by experienced radiologists as requiring or not requiring clinical follow‐up; reporting of incidental findings in clinical reports for treating physicians (two Australian sites only).
Results: A total of 4403 participants completed baseline LDCT screening at the six participating hospitals. The mean age (64–65 years) and the proportions of participants who currently smoked (47–55%) were similar at all six sites; the proportion of female participants was larger in Sydney (52%) and Vancouver (51%) than the other sites (39–44%). At least one incidental finding was made during baseline LDCT screening of 3225 people (72.8%); findings in 454 people (10.3%) required clinical follow‐up. The most frequent incidental findings were coronary artery calcification (3022 of 4380 participants with recorded results, 69.0%) and emphysema (2378 of 4401, 54.0%). Marked differences between the Australian and Canadian sites in the prevalence of incidental findings were noted, and also between the two Australian sites in their communication of incidental findings in clinical screening reports.
Conclusion: Incidental findings during lung cancer screening were frequent, and clinical reporting of these findings was inconsistent. When LDCT lung cancer screening is introduced in Australia, a standardised reporting template should be used to provide clear guidance about the clinical significance of such findings.
Trial registration: ClinicalTrials.gov, NCT02871856 (prospective, 18 August 2016).