Clinical record
In August 2022, a 19‐year‐old male student with a history of asthma and nut anaphylaxis was taken by ambulance to hospital when he was found unresponsive with vomitus and faecal soiling after one day of lethargy and sore throat. On arrival, his blood pressure was unrecordable, heart rate was 110 beats per minute, respiratory rate 55 breaths per minute, oxygen saturation 63%, temperature 36°C, and the Glasgow Coma Scale score was 6. He had no neurological signs, no meningism and no rash, and was up to date with immunisations, including meningococcal C and ACWY vaccinations.
Investigations revealed hyperlactataemia 7.8 mmol/L (reference interval [RI], < 2 mmol/L), neutrophilia 27 × 109/L (RI, 1.7–7 × 109/L), anaemia 104 g/L (RI, 130–180 g/L), thrombocytopenia 124 × 109/L (RI, 150–450 × 109/L), C‐reactive protein (CRP) 233 mg/L (RI, < 3.0 mg/L), bilirubin 25 μmol/L (RI, < 20 μmol/L), and international normalised ratio 2 (RI, 0.8–1.3), precluding lumbar puncture. Serum creatinine and transaminases were normal. A nasopharyngeal swab for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), influenza virus and respiratory syncytial virus by polymerase chain reaction (PCR), and pneumococcal and Legionella urinary antigens were negative. He was treated for septic shock with intravenous antibiotics (flucloxacillin, gentamicin), hydrocortisone, noradrenaline, and fluids, and was intubated and admitted to the intensive care unit. Computed tomography revealed bilateral pulmonary ground glass opacities. On infectious diseases consultation, toxin‐mediated shock and meningococcal aetiology was suspected, which prompted droplet precautions, public health unit notification, and a change in antibiotics to intravenous ceftriaxone and clindamycin. Gram‐negative diplococci were isolated from blood culture at 17 hours and were positive for Neisseria meningitidis serogroup B on blood PCR (ctrA and porA genes). Clinical improvement led to extubation 30 hours after admission. The patient experienced retrograde amnesia with otherwise full neurological recovery and resolution of fevers by day 4. Treatment was completed with five days of ceftriaxone.
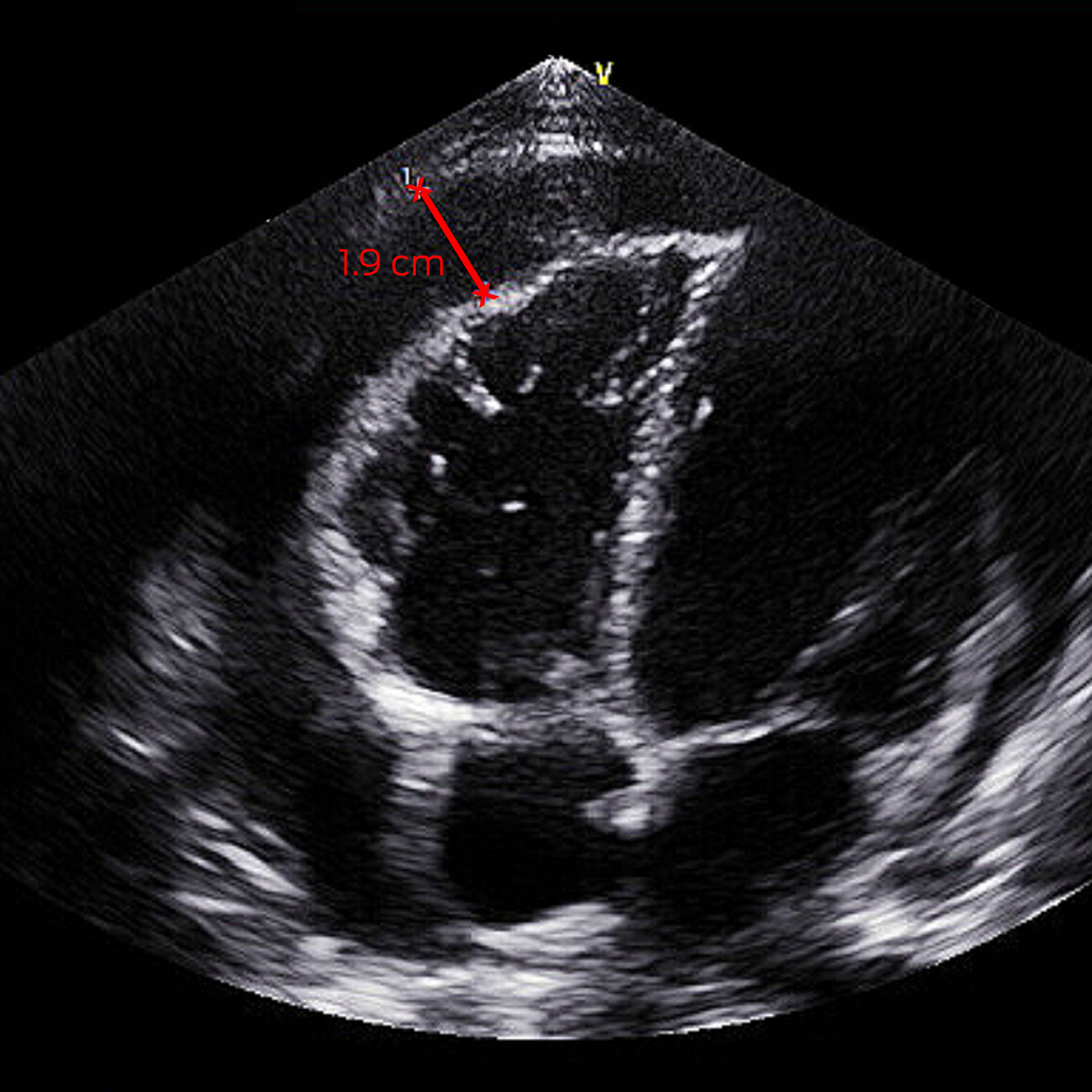
On day 5, his fevers recrudesced to 39°C with a new pericardial rub and anterior T‐wave flattening on electrocardiogram. Investigations demonstrated elevated troponin T 193 ng/L (RI, < 14 ng/L), CRP 65 mg/L, and total haemolytic complement > 1000 U/mL (RI, < 1000 U/mL). Septic screen was negative. Transthoracic echocardiography (TTE) showed a 0.5 cm pericardial effusion increasing to 1.9 cm (Box 1). He was treated for reactive meningococcal pericarditis with regular ibuprofen and colchicine, with resolution of fevers and improvement in inflammatory markers, and he was discharged five days later. He had negative human immunodeficiency virus (HIV) serology and normal complement studies after recovery. Minimal effusion on TTE by week 6 prompted transition to colchicine monotherapy, which was ceased at week 10.
The public health unit initiated contact tracing on clinical suspicion of invasive meningococcal disease (IMD) before microbiological confirmation. Seven higher risk contacts were identified and received ciprofloxacin clearance antibiotics and information on IMD. Information was provided to lower risk contacts. No further cases of IMD were identified.
Discussion
IMD is caused by Neisseria meningitidis, an aerobic gram‐negative diplococcus that colonises the human nasopharynx. Transmission occurs by respiratory droplets and prolonged exposure with asymptomatic carriers. Pathogenic strains produce a polysaccharide capsule that inhibits complement‐mediated lysis, enhancing intracellular survival and bloodstream invasion.1 Incubation is one to seven days and rarely up to ten days, followed by infection ranging from mild respiratory illness to IMD with septic shock, suppurative meningitis, or both. Secondary disseminated intravascular coagulopathy produces a petechial rash, which can proceed to tissue infarction.2 Diagnosis is confirmed by PCR test or microscopy and culture of N. meningitidis from a sterile site.2 Presentations may be atypical and lack overt meningism or rash, as in this case.
IMD occurs seasonally during winter and spring in a bimodal age distribution, with highest incidence in children aged under five years and adolescents aged 15–19 years.2 Risk factors include immunosuppression, terminal complement pathway deficiencies, smoking, and recent upper respiratory tract infections with higher rates in Aboriginal and Torres Strait Islander people.1,2,3 IMD is associated with 10% mortality, with 10–20% of survivors having long term complications.2 Autoinflammatory complications from type III hypersensitivity reactions, notably pericarditis, arthritis, pleuritis and vasculitis with secondary fever, can occur between day 4 and 14 during the recovery phase.1,2 Unrecognised, outcomes for reactive meningococcal pericarditis can be poor. Treatment includes non‐steroidal anti‐inflammatories, colchicine, and, occasionally, corticosteroids and pericardiocentesis.1
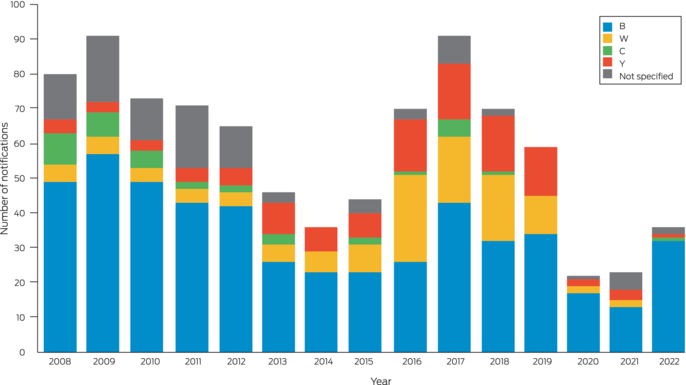
In Australia, IMD is clinician‐ and laboratory‐notifiable on a high index of suspicion. It is rare, with annual incidence decreasing from 3.5 cases per 100 000 in 2002 to 0.8 per 100 000 population in 2019, with serogroups B, C, W and Y causing the majority of cases.3,4 In 2018 and 2019, serogroup ACWY vaccination was added to the National Immunisation Program for infants and adolescents. This followed initial jurisdictional‐level programs in response to serogroup W outbreaks and increases in serogroup Y disease. Serogroup B has since become predominant nationally and in New South Wales (Box 2).5 In 2020, meningococcal B vaccination was added to the National Immunisation Program for Indigenous children and people with medical conditions associated with increased risk of IMD.3
Incidence further declined to 0.3 per 100 000 population in 2021,6 likely from measures to reduce SARS‐CoV‐2 transmission.4 Minimal influenza virus transmission likely also contributed, as antecedent influenza infection is a risk factor for IMD.4 With the relaxation of measures related to the coronavirus disease 2019 (COVID‐19) pandemic, the threat of IMD is re‐emerging. This case highlights the importance of early diagnosis, prompt public health notification, and recognition of atypical presentations and serious sequelae such as pericarditis.
Lessons from practice
- Invasive meningococcal disease (IMD) is re‐emerging after the relaxation of coronavirus disease 2019 (COVID‐19) public health measures.
- IMD may present atypically without rash or neurological signs.
- Recovery from IMD may be complicated by autoreactive sequelae, including pericarditis.
- Early clinical recognition and public health notification is imperative for the management of IMD.
- Meningococcal B vaccine is free under the National Immunisation Program for Aboriginal and Torres Strait Islander children under two years of age, and people of all ages with asplenia and hyposplenia, complement deficiency, and those receiving treatment with eculizumab.
Box 1 – Transthoracic echocardiogram off‐axis four chamber view with pericardial effusion measuring 1.9 cm
Box 2 – Invasive meningococcal disease notifications in New South Wales residents, by year of onset and serotype (2008–2022)5
Provenance: Not commissioned; externally peer reviewed.
- 1. Pace D, Pollard AJ. Meningococcal disease: clinical presentation and sequelae. Vaccine 2012; 30: B3‐B9.
- 2. Communicable Diseases Network Australia. Invasive meningococcal disease CDNA national guidelines for public health units. Canberra: CDNA, 2017. https://www.health.gov.au/sites/default/files/documents/2020/02/invasive‐meningococcal‐disease‐cdna‐national‐guidelines‐for‐public‐health‐units.pdf (viewed Nov 2022).
- 3. Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook: meningococcal disease. Canberra: Commonwealth of Australia, 2022. https://immunisationhandbook.health.gov.au/contents/vaccine‐preventable‐diseases/meningococcal‐disease (viewed Nov 2022).
- 4. George CRR, Booy R, Nissen MD, Lahra MM. The decline of invasive meningococcal disease and influenza in the time of COVID‐19: the silver linings of the pandemic playbook. Med J Aust 2022; 216: 504‐507. https://www.mja.com.au/journal/2022/216/10/decline‐invasive‐meningococcal‐disease‐and‐influenza‐time‐covid‐19‐silver
- 5. NSW Health. Notifiable Conditions Information Management System (NCIMS), Communicable Diseases Branch and Centre for Epidemiology and Evidence. Sydney: NSW Government, 2022. https://www.health.nsw.gov.au/infectious/pages/data.aspx (viewed Nov 2022).
- 6. Lahra MM, George R, Hogan TR. Australian Meningococcal Surveillance Programme annual report, 2021. Commun Dis Intell 2022; 46: 14.






Patient consent
The patient gave written consent for publication.
Open access:
Open access publishing facilitated by University of New South Wales, as part of the Wiley – University of New South Wales agreement via the Council of Australian University Librarians.
No relevant disclosures.