The known: Awareness among women that alcohol is a major modifiable risk factor for breast cancer is low. Brief alcohol interventions are acceptable to women attending breast screening services, but their effectiveness has not been established.
The new: Awareness of alcohol as a breast cancer risk factor, and alcohol literacy in general, improved to a greater extent among women who received a brief alcohol intervention than among women who received general lifestyle advice only.
The implications: Brief alcohol interventions can be provided in diverse clinical settings, reaching people in groups not generally recognised as being at risk of harmful drinking.
Alcohol is a major modifiable risk factor for breast cancer in women; an estimated 4.4% of new cases worldwide1 and nearly 10% of all breast cancer‐related deaths2 are attributable to alcohol consumption. In Australia, 6.6% of cases in post‐menopausal women3 and about 18% of breast cancer‐related deaths2 are attributable to alcohol consumption. However, it is concerning that awareness of this risk is low,4 particularly as increased risk is now associated with substantially lower alcohol consumption levels than those previously deemed safe.5
Alcohol consumption at any point in life increases the risk of breast cancer, but recent use, in particular, influences breast cancer risk, especially for women over 40 years of age.6 While alcohol consumption is declining in Australia, long term risky drinking (two or more standard drinks per day) has increased among women aged 40 years or more.7,8 Increases in alcohol consumption by middle‐aged and older women have also been reported overseas,9 but alcohol drinking in these age groups is not targeted by large scale breast cancer prevention programs.
Brief alcohol interventions are recommended by the recently updated Australian guidelines for the treatment of alcohol problems.10 Brief interventions can improve alcohol literacy and reduce consumption when delivered in general practice.11,12 However, barriers include the need for longer general practitioner consultations, inadequate training, and treatment services not being readily available when advice or referral is required. Other health care professionals could also be involved in responding to harmful alcohol consumption and reducing alcohol‐related harms.13 Organisations that implement population‐based breast screening programs are uniquely positioned to provide large numbers of women with timely and targeted health information and behaviour change strategies for improving alcohol literacy and reducing alcohol consumption.
The objective of our study was to assess the effectiveness of a brief alcohol intervention, Health4Her, for improving awareness of alcohol as a breast cancer risk factor, improving alcohol literacy, and reducing alcohol consumption by women aged 40 years or more attending routine breast screening.
Method
We undertook a single‐site, parallel group, double‐blinded randomised controlled trial. Assessments were undertaken in the clinic at baseline (t0) and by telephone four weeks (t1) and twelve weeks (t2) later. The trial protocol was prospectively registered with ClinicalTrials.gov (NCT04715516; 20 January 2021). Our reporting of the study conforms with the Consolidated Standards of Reporting Trials (CONSORT) guidelines.14
Participants
The study was conducted at Maroondah BreastScreen (Eastern Health, Melbourne), part of the national breast cancer screening program. Women aged 40 years or more with or without a history of breast cancer who attended the clinic for routine mammography on Tuesdays or Fridays during 5 February – 31 August 2021 and reported any alcohol consumption were invited to take part after their screening appointment. Women with hearing impairment that prohibited telephone assessment, who were not able to read or comprehend English adequately for participation, or who were pregnant (making them ineligible for breast screening) were not invited to participate. To conceal the alcohol focus of the study and to blind participants to trial arm allocation, they were told that the study compared two types of women's health promotion. Data collected for the study were managed with the web‐based Research Electronic Data Capture (REDCap) application.15 Follow‐up data collection ended on 2 December 2021.
Randomisation and blinding
Following eligibility screening, participants were randomly assigned to the active (brief alcohol intervention) or control arm of the study (1:1) using standard computer‐generated permuted blocks of variable size; the data scientist who generated the random allocation sequence played no other role in the study. The on‐site researcher responsible for recruitment, baseline assessments, and delivering the intervention was not blind to treatment assignment; the participating women and the researcher who undertook follow‐up assessments were blinded to treatment assignment.
Interventions
The intervention was delivered after the participant's breast screening appointment. The prototype brief e‐health intervention included alcohol‐related questions asked by the researcher and an animation viewed on an iPad (activated by the researcher), using earphones for private viewing. The online survey platform that hosted the intervention (Qualtrics) registered the time participants spent viewing the animation as a measure of intervention completion. After watching the animation, participants were directed to the service exit without the opportunity to discuss it with other participants.
Health4Her intervention
For participants in the active arm, the animation included four minutes of brief alcohol intervention and three minutes of lifestyle health promotion (physical activity, maintaining a healthy weight). They were given a takeaway pamphlet summarising the alcohol information in the animation (Supporting Information, part 1), and one on nutrition for maintaining a healthy weight.16
The Health4Her intervention was developed in accordance with brief alcohol intervention principles,11,17 and applied behaviour change approaches18,19,20,21 and findings from a pre‐implementation study of women attending breast screening services.22 Participant responses to baseline questions that assessed whether their current alcohol use exceeded national guideline recommendations determined which of two versions of the Health4Her animation they saw; both versions included personalised feedback and comparison with gender‐ and age‐specific drinking norms (Supporting Information, part 2), negative messages about the risks and harms of alcohol use (particularly the link between alcohol use and breast cancer), positive messages about the health benefits of reducing alcohol use (particularly for reducing breast cancer risk), and alcohol harm reduction strategies.
Control intervention
For participants in the control arm, the animation included three minutes of lifestyle health promotion focused on physical activity and maintaining a healthy weight for reducing breast cancer risk. They also received the pamphlet on nutrition for maintaining a healthy weight.16
Outcomes
The primary outcome was change in the proportion of participants who identified alcohol as a clear risk factor for breast cancer, consistent with Cancer Australia definitions of breast cancer risk factors,23 assessed four weeks after the intervention with a scaled response question. Secondary outcomes were change in alcohol literacy (week 4; multiple choice and free response questions) and change in alcohol consumption (weeks 4 and 12) assessed using timeline follow‐back.24 Pre‐specified exploratory outcomes were change in knowledge of other breast cancer risk factors (week 4; scaled response items) (Supporting Information, part 3). Results for other planned outcomes (program implementation evaluation, including change in general health, quality of life, and views on alcohol use and breast cancer risk) will be reported elsewhere.
Sample size calculation
We estimated that 22% of participants would identify alcohol use as a clear risk factor for breast cancer at baseline (based on the findings of our pre‐implementation study22) and that the proportion in the active intervention arm would be at least 12 percentage points larger four weeks after the intervention (based on the findings of a similar English study25). A sample of 548 participants (allowing 20% attrition) would provide 80% power to reject the null hypothesis for the primary outcome (two‐tailed, α = 0.05).
Statistical analyses
Data analyses were conducted in Stata 17.0. We examined outcomes (relative to baseline) four weeks, twelve weeks, or four and twelve weeks after the intervention (period pre‐specified by outcome). Data for all participants (apart from one who requested that their data be withdrawn) were included in analyses (intention‐to‐treat). Treatment effect for all outcomes was assessed in generalised linear mixed models with fixed effects for treatment, time, and their interaction, with participant and multiple assessments within participants as random effects. For alcohol consumption outcomes, we undertook subgroup analyses for participants who reported exceeding national guidelines for weekly consumption. Mixed models are robust to assumptions that data are missing at random or completely at random,26 consistent with intention‐to‐treat principles. Effects are reported as odds ratios (ORs) with 95% confidence intervals (CIs). For outcomes assessed at baseline and four weeks, we undertook Cochran–Mantel–Haenszel tests. P < 0.05 was deemed statistically significant (two‐tailed).
Ethics approval
The Eastern Health (LR19/011/50551) and Monash University (21395) human research ethics committees approved the study. Participants provided verbal or written informed consent to participation.
Results
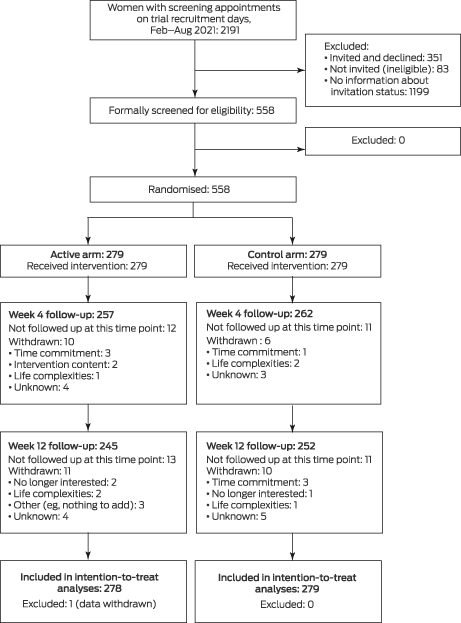
Of 2191 women who attended Maroondah BreastScreen for a screening appointment on trial recruitment days during 5 February – 31 August 2021, 558 were invited by breast screening staff to participate in the study and agreed to do so (25.5%); 351 were invited and declined (16.0%), 83 were not invited after exclusion during the pre‐screening process (3.8%), and no information on whether they were invited was recorded for 1199 women (54.7%). Of those who agreed to participate, 279 were randomly assigned to the active arm (one of whom subsequently withdrew their data from the study) and 279 to the control arm. The mean age of the participants was 60.3 years (standard deviation, 7.7 years; range, 40–87 years); 455 had recently consumed alcohol (82%). At week 4, 257 active arm (92%) and 262 control arm participants (94%) were followed up; at week 12, 245 active arm (88%) and 252 control arm participants (90%) were followed up (Box 1, Box 2).
Primary outcome
The proportions of participants who identified alcohol as a clear risk factor for breast cancer were larger at four weeks than at baseline for both the active (65% v 20%; OR, 41 [95% CI, 18–97]) and control arms (38% v 20%; OR, 4.9 [95% CI, 2.8–8.8]). The difference in change over time between the two study arms was statistically significant (arm × time: P < 0.001) (Box 3, Box 4; Supporting Information, table 1).
Secondary and pre‐specified exploratory outcomes
The increase in alcohol literacy between baseline and week 4 was larger in the active than in the control arm for each of the three secondary outcome items and for identifying alcohol as a risk factor (exploratory outcome: free response item) (Box 3, Box 5; Supporting Information, table 1).
Most changes in alcohol consumption outcomes between baseline and week 4 or week 12 were similar for the two study arms, and not statistically significant for either arm (Box 6, Box 7). The difference in change over time in number of days during the past fortnight on which more than four standard drinks were consumed was statistically significant (arm × time: P = 0.026), but the changes in each arm were not (Box 7). An analysis restricted to the 73 participants whose consumption exceeded national guidelines for weekly alcohol consumption at baseline (control arm, 38 women; active arm, 35 women) yielded similar results (Supporting Information, tables 4 and 5).
The increases between baseline and week 4 in the proportions of participants who identified inactivity and excess weight as breast cancer risk factors (information provided in both study arms) was statistically significant for both arms, but the difference between arms was not (Box 3, Box 8; Supporting Information, table 1).
Discussion
We report the first randomised controlled trial of a brief alcohol intervention for women attending a breast screening service. At baseline, 20% of participants were aware that alcohol use was a risk factor for breast cancer. The proportion who identified this link four weeks after the Health4Her intervention (65%) was larger than for the control group (38%). The increase in alcohol literacy (knowledge of the alcohol content of a standard drink, the number of standard drinks in an average restaurant serve of red wine, and recommended maximum number of standard drinks per week) was also greater following the Health4Her intervention. These results are consistent with other reports that brief e‐health interventions are effective for increasing alcohol‐related knowledge.28
Our findings indicate that our intervention could be an important first step to increasing alcohol literacy and reducing alcohol consumption by a group of people not previously targeted by alcohol‐related health education. The Health4Her intervention did not reduce alcohol consumption overall, in contrast to the consistent, if relatively small, effects of brief interventions for reducing consumption by people attending primary care clinics.11 Brief intervention trials typically include only people who consume alcohol at hazardous levels, but we recruited women regardless of their drinking level, allowing us to provide a brief alcohol intervention in a discrete, non‐stigmatising manner. However, including non‐drinkers and women drinking at lower levels may have limited the capacity of the intervention to change alcohol consumption; advice based on current Australian guidelines may not have reduced consumption by women already drinking at a low‐risk level. Larger studies of the effectiveness of Health4Her for reducing alcohol consumption are desirable, but the information content of the intervention also requires consideration. As breast cancer is the only cancer for which incidence increases with even very light levels of alcohol consumption, there may be no safe drinking level.5
We found that our tailored brief intervention improved awareness of the link between alcohol use and breast cancer risk. Alcohol literacy is necessary but not sufficient for behavioural change. Two theoretical frameworks describe the complex translation of awareness into the modification of behaviour. In the transtheoretical model, knowledge is a component of the first of three stages that precede behavioural change (pre‐contemplation), followed by contemplation (weighing up the benefits and costs of change) and preparation (committing to and planning for change).29 According to social cognitive theory, even when someone knows what they should do, their behaviour is influenced by factors that include perceived self‐efficacy, or belief in their ability to undertake what is needed, which in turn is influenced by previous success in altering behaviour, the behaviour of people around them, social persuasion (eg, “you can do it” messages), and their mood states.30
The line from knowledge to behaviour is consequently not straight, and a multifaceted strategy is needed to take the numerous cognitive and social influences on alcohol‐related behaviour and behaviour change into account. For example, the marked decline in smoking rates in many countries is attributable to the sustained, combined effects of targeted programs, tobacco control measures (eg, taxation, advertising bans, smoke‐free policies), and media campaigns.31 Harmful alcohol consumption is a causal factor for more than two hundred diseases and conditions;2,32 most health professionals and services consequently have the opportunity to increase alcohol risk awareness and motivate behavioural change as part of their routine practice.
Finally, we found that the lifestyle information provided to participants in both the active and control arms of our trial was effective for increasing awareness of inactivity and excess weight as modifiable breast cancer risk factors. This added benefit of the Health4Her intervention justifies providing it to all women who attend breast screening, including those who do not drink alcohol.
Limitations
Both the participating women and the researcher who undertook follow‐up assessments were blinded to treatment assignment in our study; a typical limitation of studies of general practice‐based brief alcohol intervention for people drinking at hazardous levels is bias associated with difficulties in participant blinding.33 Our eligibility criteria were non‐restrictive, but our study was conducted at a single breast screening clinic, possibly limiting the generalisability of our findings. Most estimated differences in alcohol and breast cancer literacy outcomes favoured the active intervention, but positive changes were generally evident in both arms. Control group response is frequently reported in alcohol intervention research, whereby assessment reactivity (exposure to alcohol questions alone prompting awareness or behavioural self‐regulation) can lead to underestimation of the effectiveness of active interventions.34 For alcohol consumption outcomes, we relied on participant reports, which may be inaccurate because of recall bias or perceptions of social acceptability. Finally, Health4Her improved knowledge, a critical element of behavioural change, but further development of the intervention according to contemporary behavioural models and larger trials of the intervention are still required.
Conclusion
The Health4Her intervention was effective in improving awareness of alcohol as a breast cancer risk factor among women who attended a breast screening service, and also improved alcohol literacy more broadly. The effectiveness of brief alcohol interventions for reducing alcohol consumption in women attending breast screening should be further investigated. Our targeted approach in a novel clinical setting to reducing harmful alcohol consumption, complementing any screening and brief alcohol interventions in general practice, could be widely implemented elsewhere. It is particularly relevant in view of the increasing prevalence of risky drinking among middle‐aged and older women, and strong evidence that even very light alcohol consumption increases breast cancer risk.
Data sharing statement
The data for this study will not be shared, as we do not have permission from the participants or ethics approval to do so.
Box 1 – CONSORT flow diagram of the invitation, selection, and screening of women for participation in the Health4Her brief alcohol intervention trial
Box 2 – Socio‐demographic characteristics of the women who participated in the Health4Her brief alcohol intervention trial
|
Characteristic |
All participants* |
Control arm |
Intervention arm |
||||||||||||
|
|
|||||||||||||||
|
Participating women |
557 |
279 |
278 |
||||||||||||
|
Age (years), mean (SD) |
60.3 (7.7) |
60.7 (8.0) |
59.8 (7.3) |
||||||||||||
|
Born in Australia |
433 (78%) |
210 (75%) |
223 (80%) |
||||||||||||
|
Geographic area (residence)27 |
|
|
|
||||||||||||
|
Major city |
540 (97%) |
273 (98%) |
267 (96%) |
||||||||||||
|
Other |
17 (3%) |
6 (2%) |
11 (4%) |
||||||||||||
|
Culturally and linguistically diverse background |
72 (13%) |
36 (13%) |
36 (13%) |
||||||||||||
|
LGBTIQA+ identity |
8 (1%) |
5 (28%) |
3 (1%) |
||||||||||||
|
Education level |
|
|
|
||||||||||||
|
Did not complete year 12 |
105 (19%) |
50 (18%) |
55 (20%) |
||||||||||||
|
Year 12 or equivalent |
74 (13%) |
39 (14%) |
35 (13%) |
||||||||||||
|
Vocational training/apprenticeship |
86 (15%) |
44 (16%) |
42 (15%) |
||||||||||||
|
Diploma, advanced diploma, associate degree |
91 (16%) |
51 (18%) |
40 (14%) |
||||||||||||
|
Bachelor's degree |
162 (29%) |
77 (28%) |
85 (31%) |
||||||||||||
|
Postgraduate degree |
39 (7%) |
18 (6%) |
21 (8%) |
||||||||||||
|
Household |
|
|
|
||||||||||||
|
Living alone |
79 (14%) |
41 (15%) |
38 (14%) |
||||||||||||
|
Living with partner or spouse |
400 (72%) |
202 (72%) |
198 (71%) |
||||||||||||
|
Living with own children (dependants) |
86 (15%) |
43 (15%) |
43 (16%) |
||||||||||||
|
Living with other dependants requiring care |
14 (2%) |
9 (3%) |
5 (2%) |
||||||||||||
|
Living with own children (adults) |
155 (28%) |
71 (25%) |
84 (30%) |
||||||||||||
|
Living with other adults (relative/non‐relative) |
32 (6%) |
24 (9%) |
8 (3%) |
||||||||||||
|
Other |
8 (1%) |
3 (1%) |
5 (2%) |
||||||||||||
|
Typical alcohol drinking frequency |
|
|
|
||||||||||||
|
Every day |
31 (6%) |
19 (7%) |
12 (4%) |
||||||||||||
|
5–6 days per week |
43 (8%) |
22 (8%) |
21 (8%) |
||||||||||||
|
3–4 days per week |
92 (16%) |
44 (16%) |
48 (17%) |
||||||||||||
|
1–2 days per week |
107 (19%) |
54 (19%) |
53 (19%) |
||||||||||||
|
2–3 days per month |
78 (14%) |
40 (14%) |
38 (14%) |
||||||||||||
|
1 day per month |
43 (8%) |
22 (8%) |
21 (8%) |
||||||||||||
|
Occasionally, not in the past month |
61 (11%) |
28 (10%) |
33 (12%) |
||||||||||||
|
No longer drink or never drank |
102 (18%) |
50 (18%) |
52 (19%) |
||||||||||||
|
Alcohol consumption during preceding fortnight (timeline follow‐back) |
|
|
|
||||||||||||
|
Alcohol consumed (days), mean (SD) |
3.2 (3.9) |
3.4 (4.2) |
3.0 (3.7) |
||||||||||||
|
More than two standard drinks consumed (days), mean (SD) |
1.5 (2.9) |
1.5 (2.9) |
1.5 (2.9) |
||||||||||||
|
More than four standard drinks consumed (days), mean (SD) |
0.4 (1.7) |
0.3 (1.5) |
0.6 (1.9) |
||||||||||||
|
Total standard drinks, mean (SD) |
7.8 (13.0) |
7.5 (11.3) |
8.1 (14.5) |
||||||||||||
|
Exceeding Australian alcohol guidelines (weekly consumption)10 |
73 (13%) |
38 (14%) |
35 (13%) |
||||||||||||
|
Exceeding Australian alcohol guidelines (daily consumption)10 |
71 (13%) |
31 (11%) |
40 (14%) |
||||||||||||
|
|
|||||||||||||||
|
LGBTIQA+ = lesbian, gay, bisexual, transgender, intersex, queer, asexual, and other non‐binary sexual and gender identities; SD = standard deviation. * Of the 558 participants allocated to the active intervention or control groups, one intervention arm participant subsequently withdrew their data from the study. |
|||||||||||||||
Box 3 – Primary, secondary and pre‐specified exploratory alcohol and breast cancer literacy outcomes, for 279 control and 278 active intervention arm participants (baseline) and 262 control and 257 active intervention arm participants (week 4)
|
|
Baseline |
Week 4 |
|
|
|||||||||||
|
Characteristic |
Number |
Proportion (95% CI) |
Number |
Proportion (95% CI) |
Odds ratio (95% CI) |
P (arm × time) |
|||||||||
|
|
|||||||||||||||
|
Primary outcome: knowledge of alcohol as a clear breast cancer risk factor (scaled response) |
|
|
|
|
|
< 0.001 |
|||||||||
|
Control arm |
55 |
20% (15–25%) |
99 |
38% (32–44%) |
4.9 (2.8–8.8) |
|
|||||||||
|
Active arm |
55 |
20% (16–25%) |
168 |
65% (59–71%) |
41 (18–97) |
|
|||||||||
|
Secondary outcomes: alcohol literacy |
|
|
|
|
|
|
|||||||||
|
Alcohol in a standard drink* |
|
|
|
|
|
< 0.001 |
|||||||||
|
Control arm |
32 |
12% (8.3–16%) |
24 |
9.2% (6.2–13%) |
0.7 (0.4–1.4) |
|
|||||||||
|
Active arm |
30 |
11% (7.7–15%) |
60 |
23% (19–29%) |
3.7 (2.0–6.9) |
|
|||||||||
|
Standard drinks in an average restaurant serve of red wine |
|
|
|
|
|
0.006 |
|||||||||
|
Control arm |
16 |
5.7% (3.5–9.2%) |
17 |
6.5% (4.1–10%) |
1.2 (0.5–2.7) |
|
|||||||||
|
Active arm |
22 |
7.9% (5.3–12%) |
57 |
22% (17–28%) |
5.1 (2.6–9.9) |
|
|||||||||
|
Maximum weekly consumption recommended by Australian Alcohol Guidelines† |
|
|
|
|
|
0.042 |
|||||||||
|
Control arm |
7 |
2.5% (1.2–5.2%) |
9 |
4.8% (2.5–9.0%) |
2.6 (0.6–10) |
|
|||||||||
|
Active arm |
11 |
4.0% (2.2–7.0%) |
31 |
16% (12–22%) |
17 (4.2–68) |
|
|||||||||
|
Pre‐specified exploratory outcomes: knowledge of breast cancer risk factors |
|
|
|
|
|
|
|||||||||
|
Alcohol as a breast cancer risk factor (free response)‡ |
|
|
|
|
|
< 0.001 |
|||||||||
|
Control arm |
64 |
23% (18–28%) |
123 |
47% (41–53%) |
5.6 (3.3–9.6) |
|
|||||||||
|
Active arm |
54 |
19% (15–25%) |
170 |
66% (60–72%) |
28 (14–58) |
|
|||||||||
|
Inactivity as a clear breast cancer risk factor |
|
|
|
|
|
|
|||||||||
|
Control arm |
20 |
7.2% (4.7–11%) |
87 |
33% (28–39%) |
15 (7.0–32) |
|
|||||||||
|
Active arm |
30 |
11% (7.6–15%) |
83 |
32% (27–38%) |
7.9 (4.1–15) |
|
|||||||||
|
Excess weight as a clear breast cancer risk factor |
|
|
|
|
|
0.61 |
|||||||||
|
Control arm |
62 |
22% (18–28%) |
138 |
53% (47–59%) |
8.3 (4.8–14) |
|
|||||||||
|
Active arm |
71 |
26% (21–31%) |
137 |
53% (47–59%) |
7.0 (4.0–12) |
|
|||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Missing baseline data: three participants from each arm. † Missing week 4 data: 65 participants in active arm, 74 participants in control arm. ‡ Missing baseline data: one participant from the control arm. |
|||||||||||||||
Box 4 – Primary outcome: proportions of participants who identified alcohol as a clear breast cancer risk factor (scaled response)*
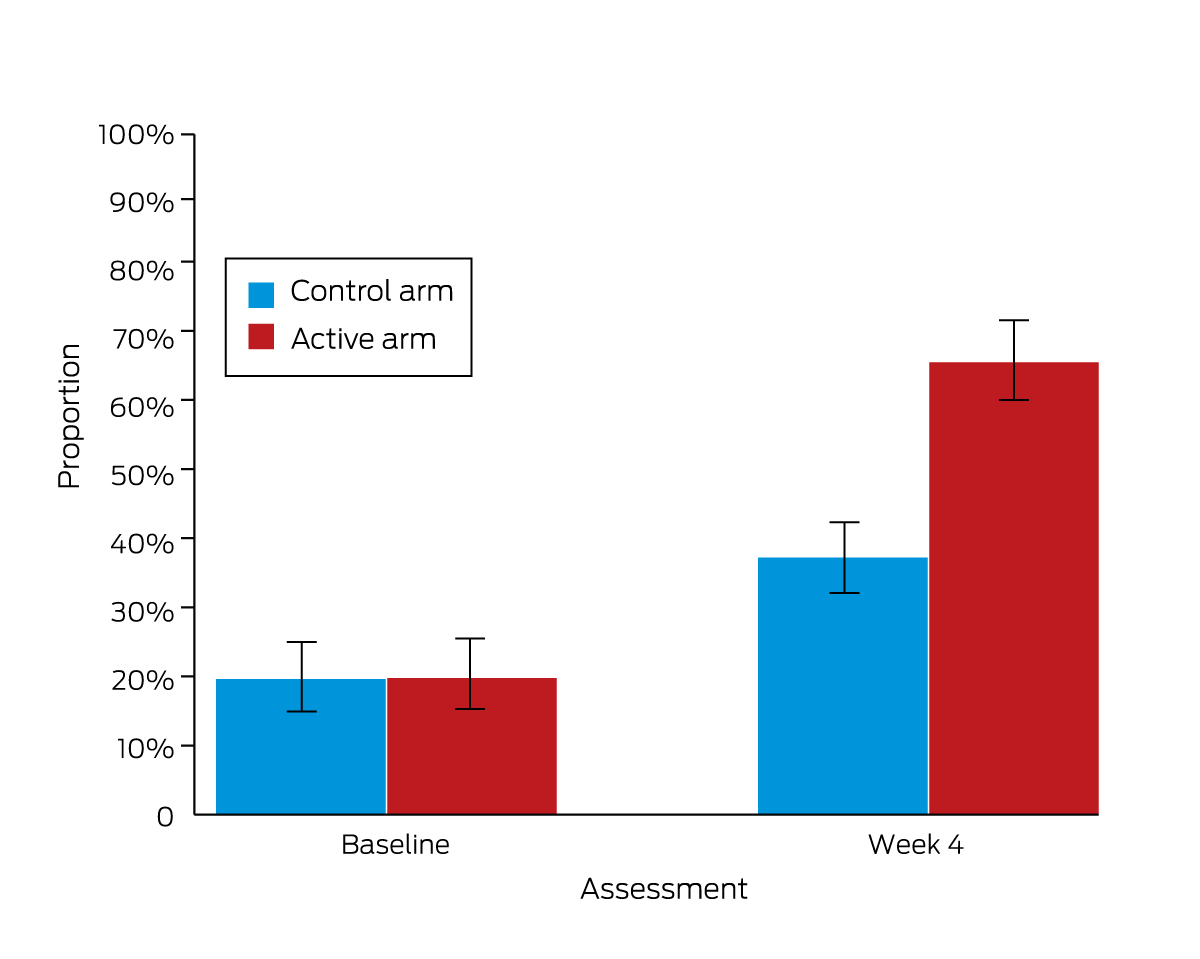
* The data for this graph are included in Box 3.
Box 5 – Secondary (alcohol literacy) and pre‐specified exploratory (alcohol as a risk factor: free response) outcomes*
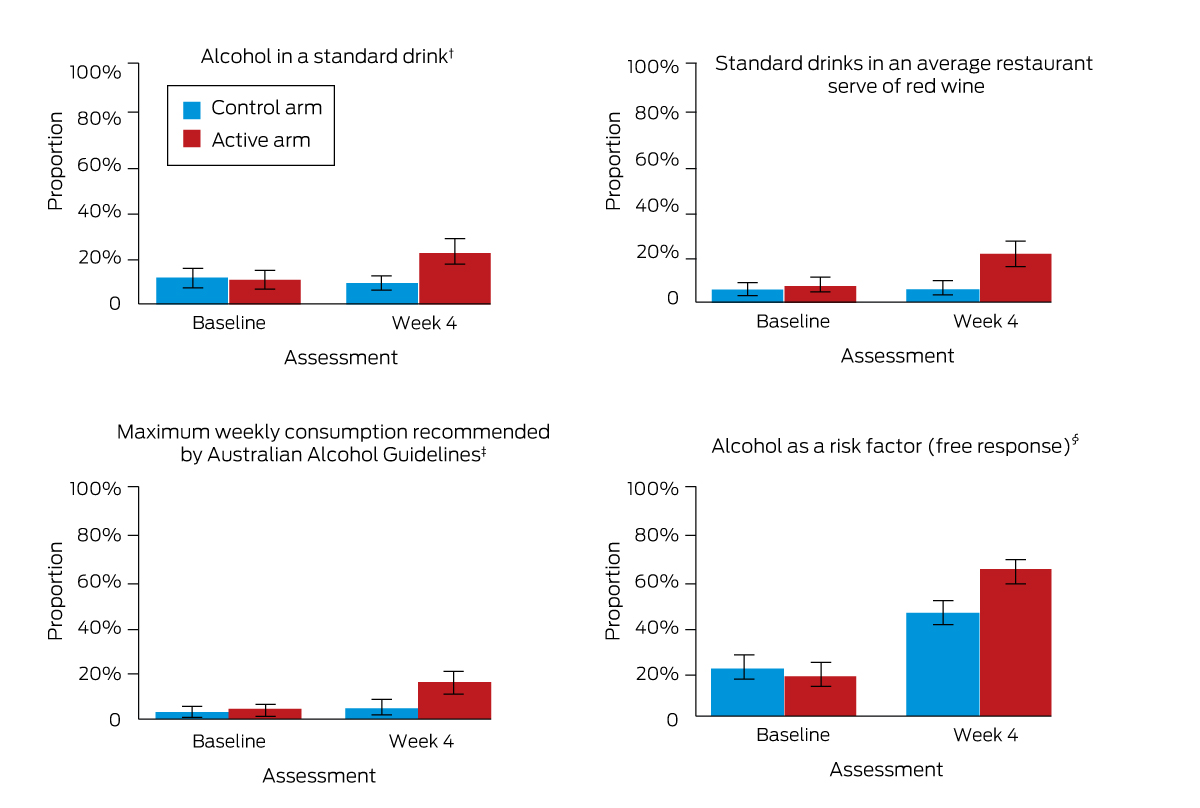
* The data for this graph are included in Box 3. † Missing baseline data: three participants from each arm. ‡ Missing week 4 data: 65 participants in active arm, 74 participants in control arm. § Missing baseline data: one participant from the control arm.
Box 6 – Secondary outcomes: alcohol consumption exceeding Australian alcohol consumption guideline recommendations10
|
|
Proportion (95% CI) |
Odds ratio (95% CI) |
|
||||||||||||
|
Characteristic |
Baseline (t0) |
Week 4 (t1) |
Week 12 (t2) |
t1 v t0 |
t2 v t0 |
P (arm × time) |
|||||||||
|
|
|||||||||||||||
|
More than ten standard drinks in a week on at least one of two preceding weeks |
|
|
|
|
|
0.43 |
|||||||||
|
Control arm |
14% (10–18%) |
15% (11–19%) |
13% (9.1–17%) |
1.3 (0.6–2.8) |
0.9 (0.4–1.9) |
|
|||||||||
|
Active arm |
13% (9.2–17%) |
16% (12–21%) |
13% (9.7–18%) |
2.5 (1.1–5.7) |
1.6 (0.7–3.8) |
|
|||||||||
|
More than ten standard drinks in a week during both of the two preceding weeks |
|
|
|
|
|
0.45 |
|||||||||
|
Control arm |
9.0% (6.1–13%) |
11% (7.5–15%) |
10% (7.1–15%) |
1.9 (0.7–5.6) |
1.6 (0.5–4.7) |
|
|||||||||
|
Active arm |
9.0% (6.1–13%) |
10% (7.0–14%) |
11% (8.0–16%) |
2.6 (0.9–7.9) |
4.2 (1.3–13) |
|
|||||||||
|
More than four standard drinks in a day (on at least one day) |
|
|
|
|
|
0.15 |
|||||||||
|
Control arm |
11% (7.9–15%) |
7.3% (4.7–11%) |
5.6% (3.3–9.2%) |
0.4 (0.1–1.0) |
0.2 (0.1–0.6) |
|
|||||||||
|
Active arm |
14% (11–19%) |
8.6% (5.7–13%) |
11% (7.7–16%) |
0.3 (0.1–0.8) |
0.6 (0.2–1.4) |
|
|||||||||
|
More than four standard drinks in a day (on more than one day) |
|
|
|
|
|
0.16 |
|||||||||
|
Control arm |
5.7% (3.5–9.2%) |
5.7% (3.5–9.3%) |
4.4% (2.4–7.7%) |
1.4 (0.4–5.1) |
0.7 (0.2–2.7) |
|
|||||||||
|
Active arm |
10% (7.0–14%) |
6.6% (4.1–10%) |
9.0% (6.0–13%) |
0.5 (0.1–1.8) |
1.5 (0.5–4.9) |
|
|||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Based on 14‐day timeline follow‐back. Numbers of respondents: baseline, 279 control arm, 278 active intervention; week 4, 262 control arm, 257 active intervention; week 12, 252 control arm, 245 active intervention. The number of respondents for each outcome and the number of participants who endorsed each outcome are included in the Supporting Information, table 2. |
|||||||||||||||
Box 7 – Secondary outcomes: alcohol consumption during the past fortnight*
|
|
Mean (95% CI) |
Unstandardised regression coefficient (95% CI) |
|
||||||||||||
|
Characteristic |
Baseline (t0) |
Week 4 (t1) |
Week 12 (t2) |
t1 v t0 |
t2 v t0 |
P (arm × time) |
|||||||||
|
|
|||||||||||||||
|
Days on which alcohol was consumed |
|
|
|
|
|
0.14 |
|||||||||
|
Control arm |
3.4 (2.9–3.9) |
3.4 (2.9–3.9) |
3.2 (2.7–3.7) |
0.0 (–0.3 to 0.3) |
–0.2 (–0.5 to 0.0) |
|
|||||||||
|
Active arm |
3.0 (2.6–3.5) |
3.2 (2.7–3.6) |
2.9 (2.5–3.4) |
0.3 (–0.0 to 0.5) |
0.2 (–0.1 to 0.4) |
|
|||||||||
|
Days on which more than two standard drinks were consumed |
|
|
|
|
|
0.12 |
|||||||||
|
Control arm |
1.5 (1.1–1.8) |
1.4 (1.1–1.8) |
1.7 (1.3–2.1) |
0.0 (–0.3 to 0.3) |
0.3 (–0.0 to 0.6) |
|
|||||||||
|
Active arm |
1.5 (1.2–1.8) |
1.8 (1.4–2.2) |
1.6 (1.2–2.0) |
0.4 (0.1 to 0.7) |
0.3 (0.0 to 0.6) |
|
|||||||||
|
Days on which more than four standard drinks were consumed |
|
|
|
|
|
0.026 |
|||||||||
|
Control arm |
0.4 (0.2–0.5) |
0.3 (0.1–0.5) |
0.2 (0.1–0.4) |
0.0 (–0.2 to 0.2) |
–0.1 (–0.3 to 0.1) |
|
|||||||||
|
Active arm |
0.6 (0.3–0.8) |
0.4 (0.2–0.6) |
0.6 (0.4–0.9) |
–0.1 (–0.2 to 0.1) |
0.2 (–0.0 to 0.3) |
|
|||||||||
|
Total standard drinks during fortnight |
|
|
|
|
|
0.38 |
|||||||||
|
Control arm |
7.5 (6.2–8.9) |
7.7 (6.3–9.2) |
7.3 (5.9–8.7) |
0.4 (–0.6 to 1.3) |
–0.2 (–1.1 to 0.8) |
|
|||||||||
|
Active arm |
8.1 (6.4–9.9) |
8.6 (6.8–10) |
8.1 (6.3–9.8) |
1.1 (0.1 to 2.0) |
0.7 (–0.2 to 1.7) |
|
|||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Based on 14‐day timeline follow‐back. Numbers of respondents: baseline, 279 control arm, 278 active intervention; week 4, 262 control arm, 257 active intervention; week 12, 252 control arm, 245 active intervention. The number of respondents for each outcome is included in the Supporting Information, table 3. |
|||||||||||||||
Box 8 – Pre‐specified exploratory outcomes: knowledge of breast cancer risk factors (other than alcohol use)
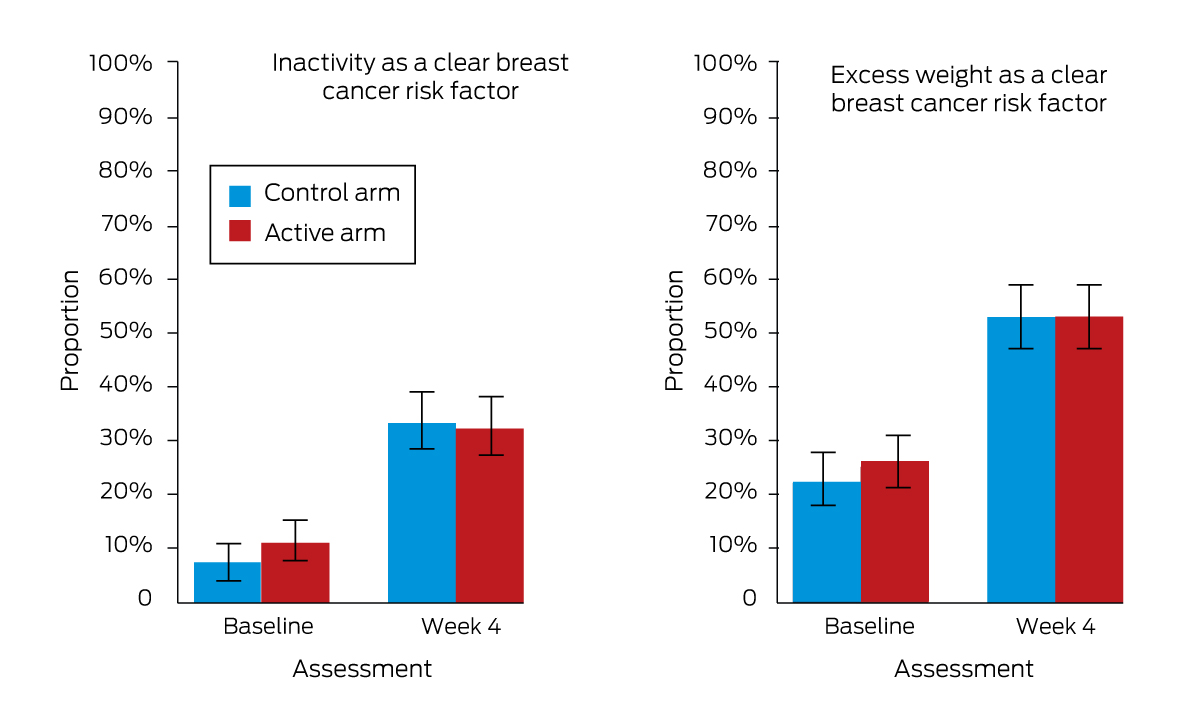
* The data for this graph are included in Box 3.
Received 22 October 2022, accepted 20 March 2023
- Jasmin Grigg1,2
- Victoria Manning1,2
- Darren Lockie3
- Michelle Giles3
- Robin J Bell4
- Peta Stragalinos1,2
- Chloe Bernard1,2
- Christopher J Greenwood5,6
- Isabelle Volpe1,2
- Liam Smith7
- Peter Bragge7
- Dan I Lubman1,2
- 1 Turning Point, Eastern Health, Melbourne, VIC
- 2 Monash Addiction Research Centre, Eastern Health Clinical School, Monash University, Melbourne, VIC
- 3 Maroondah BreastScreen, Eastern Health, Melbourne, VIC
- 4 Monash University, Melbourne, VIC
- 5 Deakin University, Geelong, VIC
- 6 Centre for Adolescent Health, Murdoch Children's Research Institute, Melbourne, VIC
- 7 Monash Sustainable Development Institute, Monash University, Melbourne, VIC
Open access:
Open access publishing facilitated by Monash University, as part of the Wiley – Monash University agreement via the Council of Australian University Librarians.
This study was supported by research grants from VicHealth and the Eastern Health Foundation. The funders had no role in any part of this study. We thank BreastScreen Victoria for their support. We thank the staff of Maroondah BreastScreen for supporting this project at their clinic, and we gratefully acknowledge all Maroondah BreastScreen clients who participated in the trial. We thank Erin Flatters (Jumbla Animation Studios) for producing the intervention animations. We thank Alun Pope (Analytical Insight) for his contribution to data preparation and statistical analyses.
Dan Lubman, Victoria Manning, Robin Bell, and Jasmin Grigg have received grants from the National Health and Medical Research Council. Dan Lubman, Victoria Manning and Robin Bell have received grants from the Medical Research Future Fund. Dan Lubman, Victoria Manning, and Jasmin Grigg have received funding from Shades of Pink and the Victorian Department of Health. Dan Lubman and Victoria Manning have received grants from the HCF Research Foundation, the Alcohol and Drug Research Innovation Agenda, the Alcohol and Drug Foundation, the Eastern Health Foundation, the Victorian Responsible Gambling Foundation, and the National Centre for Clinical Research on Emerging Drugs. Dan Lubman has received grants from Google, the Australian Research Council, VicHealth, and the Australian Department of Health and Aged Care. Victoria Manning has received funding from the Transport Accident Commission (Victoria). Jasmin Grigg has received funding from the Victorian Department of Transport and Planning. Dan Lubman is supported by a National Health and Medical Research Council Leadership Fellowship. Isabelle Volpe is supported by an Australian Government Research Training Program stipend.
- 1. Rumgay H, Shield K, Charvat H, et al. Global burden of cancer in 2020 attributable to alcohol consumption: a population‐based study. Lancet Oncol 2021; 22: 1071‐1080.
- 2. Liu H, Shi W, Jin Z, et al. Global, regional, and national mortality trends of female breast cancer by risk factor, 1990–2017. BMC Cancer 2021; 21: 459.
- 3. Arriaga ME, Vajdic CM, Canfell K, et al. The preventable burden of breast cancers for premenopausal and postmenopausal women in Australia: a pooled cohort study. Int J Cancer 2019; 145: 2383‐2394.
- 4. Sinclair J, McCann M, Sheldon E, et al. The acceptability of addressing alcohol consumption as a modifiable risk factor for breast cancer: a mixed method study within breast screening services and symptomatic breast clinics. BMJ Open 2019; 9: e027371.
- 5. Choi YJ, Myung SK, Lee JH. Light alcohol drinking and risk of cancer: a meta‐analysis of cohort studies. Cancer Res Treat 2018; 50: 474‐487.
- 6. Chen WY, Rosner B, Hankinson SE, et al. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. JAMA 2011; 306: 1884‐1890.
- 7. Australian Institute of Health and Welfare. National Drug Strategy Household Survey 2019 (Cat. no. PHE 270); Alcohol supplementary tables. 16 July 2020. https://www.aihw.gov.au/getmedia/4f178aed‐4301‐4d49‐8fe6‐c9fa663d914e/aihw‐phe‐270‐3‐Alcohol‐tables.xlsx.aspx (viewed Mar 2023).
- 8. Miller M, Mojica‐Perez Y, Livingston M, et al. The who and what of women's drinking: examining risky drinking and associated socio‐demographic factors among women aged 40–65 years in Australia. Drug Alcohol Rev 2022; 41: 724‐731.
- 9. Keyes KM, Jager J, Mal‐Sarkar T, et al. Is there a recent epidemic of women's drinking? A critical review of national studies. Alcohol Clin Exp Res 2019; 43: 1344‐1359.
- 10. Haber PS, Riordan BC, Winter DT, et al. New Australian guidelines for the treatment of alcohol problems: an overview of recommendations. Med J Aust 2021; 215 (7 Suppl): S3‐S32. https://www.mja.com.au/system/files/2021‐09/Sup_215_7_4%20Oct.pdf
- 11. Kaner EF, Beyer FR, Muirhead C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev 2018; CD004148.
- 12. Kypri K, McAnally HM. Randomized controlled trial of a web‐based primary care intervention for multiple health risk behaviors. Prev Med 2005; 41: 761‐766.
- 13. Holmwood CB. Screening and brief interventions for harmful alcohol use: where to now? Med J Aust 2021; 214: 153‐154. https://www.mja.com.au/journal/2021/214/4/screening‐and‐brief‐interventions‐harmful‐alcohol‐use‐where‐now
- 14. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332.
- 15. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95: 103208.
- 16. National Health and Medical Research Council: Department of Health and Ageing. Healthy eating for adults (N55g). 2013. www.eatforhealth.gov.au/sites/default/files/2022‐09/n55g_adult_brochure.pdf (viewed Jan 2023).
- 17. World Health Organization. Screening and brief interventions for substance use problems. Geneva: World Health Organization, 2022. www.who.int/activities/screening‐and‐brief‐interventions‐for‐substance‐use‐problems (viewed Mar 2023).
- 18. Riper H, Spek V, Boon B, et al. Effectiveness of e‐self‐help interventions for curbing adult problem drinking: a meta‐analysis. J Med Internet Res 2011; 13: e42.
- 19. Dotson KB, Dunn ME, Bowers CA. Stand‐alone personalized normative feedback for college student drinkers: a meta‐analytic review, 2004 to 2014. PLoS One 2015; 10: e0139518.
- 20. Witte K, Allen M. A meta‐analysis of fear appeals: implications for effective public health campaigns. Health Educ Behav 2000; 27: 591‐615.
- 21. Gallagher KM, Updegraff JA. Health message framing effects on attitudes, intentions, and behavior: a meta‐analytic review. Ann Behav Med 2012; 43: 101‐116.
- 22. Grigg J, Manning V, Volpe I, et al. A pre‐implementation study to understand women's knowledge of the alcohol–breast cancer link, and acceptability of alcohol brief intervention in the breast screen setting [abstract: APSAD 2021 conference, 7–10 November 2021, virtual]. Drug Alcohol Rev 40 (Suppl. 1): S75‐S76.
- 23. Cancer Australia. Breast cancer risk factors. 2019. www.breastcancerriskfactors.gov.au/risk‐factors (viewed Jan 2023).
- 24. Sobell LC, Sobell MB. Timeline follow‐back: a technique for assessing self‐reported ethanol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: psychosocial and biological methods. Totowa (NJ): Humana Press, 1992; pp. 41‐72.
- 25. Martin N, Buykx P, Shevills C, et al. Population level effects of a mass media alcohol and breast cancer campaign: a cross‐sectional pre‐intervention and post‐intervention evaluation. Alcohol Alcohol 2018; 53: 31‐38.
- 26. Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed‐model analysis. J Clin Epidemiol 2013; 66: 1022‐1028.
- 27. Australian Bureau of Statistics. 1270.0.55.005. Australian Statistical Geography Standard (ASGS). Volume 5: Remoteness structure, July 2016. 16 Mar 2018. https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/1270.0.55.005July%202016?OpenDocument (viewed Mar 2023).
- 28. Neale ZE, Salvatore JE, Cooke ME, et al. The utility of a brief web‐based prevention intervention as a universal approach for risky alcohol use in college students: evidence of moderation by family history. Front Psychol 2018; 9: 747.
- 29. DiClemente CC, Graydon MM. Changing behavior using the transtheoretical model. In: Hagger MS, Cameron LD, Hamilton K, et al, editors. The handbook of behavior change. Cambridge: Cambridge University Press, 2020; pp. 136‐149.
- 30. Bandura A. Health promotion from the perspective of social cognitive theory. Psychol Health 1998; 13: 623‐649.
- 31. Wakefield MA, Loken B, Hornik RC. Use of mass media campaigns to change health behaviour. Lancet 2010; 376: 1261‐1271.
- 32. Rehm J, Baliunas D, Borges GLG, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction 2010; 105: 817‐843.
- 33. Beyer F, Campbell F, Bertholet N, et al. The Cochrane 2018 review on brief interventions in primary care for hazardous and harmful alcohol consumption: a distillation for clinicians and policy makers. Alcohol Alcohol 2019; 54: 417‐427.
- 34. McCambridge J. Research assessments: instruments of bias and brief interventions of the future? Addiction 2009; 104: 1311‐1312.






Abstract
Objectives: To assess the effectiveness of a brief alcohol intervention for improving awareness of alcohol as a breast cancer risk factor, improving alcohol literacy, and reducing alcohol consumption by women attending routine breast screening.
Design: Single‐site, double‐blinded randomised controlled trial.
Setting: Maroondah BreastScreen (Eastern Health, Melbourne), part of the national breast cancer screening program.
Participants: Women aged 40 years or more, with or without a history of breast cancer and reporting any alcohol consumption, who attended the clinic for routine mammography during 5 February – 27 August 2021.
Intervention: Active arm: animation including brief alcohol intervention (four minutes) and lifestyle health promotion (three minutes). Control arm: lifestyle health promotion only.
Major outcome measure: Change in proportion of women who identified alcohol use as a clear risk factor for breast cancer (scaled response measure).
Results: The mean age of the 557 participants was 60.3 years (standard deviation, 7.7 years; range, 40–87 years); 455 had recently consumed alcohol (82%). The proportions of participants aware that alcohol use increased the risk of breast cancer were larger at four weeks than at baseline for both the active intervention (65%v 20%; odds ratio [OR], 41; 95% confidence interval [CI], 18–97) and control arms of the study (38% v 20%; OR, 4.9; 95% CI, 2.8–8.8), but the change over time was greater for the active intervention arm (arm × time: P < 0.001). Alcohol literacy also increased to a greater extent in the active than the control arm, but alcohol consumption did not significantly change in either arm.
Conclusion: A tailored brief alcohol intervention for women attending breast screening was effective for improving awareness of the increased breast cancer risk associated with alcohol use and alcohol literacy more broadly. Such interventions are particularly important given the rising prevalence of risky drinking among middle‐aged and older women and evidence that even very light alcohol consumption increases breast cancer risk.
Registration: ClinicalTrials.gov, NCT04715516 (prospective; 20 January 2021).