Erectile dysfunction (ED) is defined as persistent or recurrent inability to achieve and maintain a penile erection of sufficient rigidity to permit satisfactory sexual activity occurring for at least 3 months. ED has been shown to adversely affect the physical and psychosocial wellbeing and quality of life in men, their partners and families.1 It is estimated that 20–30% of adult men have experienced at least one episode of sexual dysfunction.2
The development of ED is frequently attributable to psychogenic factors and physiological alterations of neural, vascular, hormonal and endothelial function. The risk factors include older age, sedentary lifestyle, obesity, smoking, dyslipidaemia, and metabolic syndrome. These are similar to the established risk factors for cardiovascular disease,3 and the presence of ED itself serves as an important predictor marker of future cardiovascular risk, with the risks of cardiovascular disease and death increasing steadily with the severity of ED.4,5 Patients with known cardiovascular risk factors or who have a symptomatic cardiac disease should receive further investigations, with referral to cardiology where appropriate. In addition, there is a strong association between ED and lower urinary tract symptoms/benign prostatic hyperplasia.6
In Australia, most cases of ED are identified and managed by general practitioners, with specialist referrals for men who have an incomplete response or require further investigations and treatment.7 This shared care model for the treatment of ED reflects the optimal utilisation of health care resources and recognises that general practitioners play an important gatekeeper role in primary care. To our knowledge, there is currently no published peer‐reviewed Australian clinical guideline or position statement on ED that is endorsed by a major organisation. These clinical practice guidelines are endorsed by the Urological Society of Australia and New Zealand (USANZ) and the Australasian Chapter of Sexual Health Medicine (AChSHM) for the Royal Australasian College of Physicians (RACP) and provides clinicians with the current evidence‐based clinical guidance in the management of ED for Australian men.
Methods
We performed a MEDLINE literature search for English language articles, published from 2011 to 2021, using the keywords “erectile dysfunction”, “diagnosis”, “test”, “psychosexual therapy”, “phosphodiesterase inhibitor”, “intracavernosal therapy”, “penile prosthesis” and “regenerative therapy”. We also reviewed and compared published clinical guidelines from major international organisations.1,4,8,9,10,11,12,13,14,15 The panel of experts were recognised as key opinion leaders in the field of ED and were appointed as representatives from USANZ and the AChSHM for the RACP, to evaluate the clinical data concerning the management of ED with references and recommendations used in the text assessed according to their Level of evidence (LoE) based on the Oxford Centre for Evidence Based Medicine LoEs16 (Supporting Information), and the strength of the recommendations are graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system using the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument.17 There was no funding received for this consensus formulation. The panel was tasked to provide comment on the relevant headings concerning the management of ED, namely diagnostic assessment and management strategies. Any disagreement was resolved with multiple rounds of discussion and feedback. A consensus agreement was achieved using a modified Delphi method and all experts in the panel agreed on the lists of clinical recommendations. A detailed analysis of all relevant studies is not the goal of this article. These guidelines are aimed at informing clinicians on the current standard of care and providing an evidence‐based ED management endorsed by USANZ and the AChSHM for the RACP. The existing sections of the guidelines were peer reviewed, and results from ongoing and new systematic reviews will be updated in the next 5 years to incorporate new clinical findings and novel interventions.
Diagnostic assessment
Clinical history
The sexual history of the patient should ascertain the severity, onset and duration of ED; the concomitant medical or psychosocial factors; and the degree of symptom bother to the patient (and partner if applicable). If a patient has an existing sexual partner, it may be useful to have both the patient and his partner in the consultation (Expert opinion). The complaint of ED, which is mainly a problem of penile rigidity,1 should be discerned from other male sexual complaints, including libido issues, ejaculatory disorders (such as premature ejaculation), disorders of orgasm, and penile conditions (such as Peyronie disease). It is important to have an open dialogue about the patient’s sexual concerns, and the manner of sexual enquiry should reflect a high level of sensitivity and regard for everyone’s unique ethnic, cultural and personal background.15 In addition, the patient’s specific sexual goals should be elucidated and appreciated (Expert opinion). A monogamous, heterosexual relationship should not be assumed.
Potential aetiologies for ED, such as medications, stress, depression, hormonal abnormalities, tobacco consumption, excessive alcohol use, drug misuse, and partner‐specific issues, should be explored. The dichotomy between psychological and organic causes of ED is often not clear, although younger men are more likely to have psychogenic ED. However, many men with organic ED will suffer psychosexual distress, further exacerbating the state of ED.2 Cardiometabolic screening should be undertaken to stratify cardiovascular risk and identify occult cardiac disease.5,15 For older men, the severity of lower urinary tract symptoms/benign prostatic hyperplasia is closely related to ED severity,2,6,15 and treatment for one condition may beneficially or adversely affect the other.
The general domains of the clinical history include:
- determination of specifics related to ED;
- sexual desire, relationship issues, and stressors at home and work;
- lifestyle factors (such as smoking, substance use/misuse, sedentary lifestyle);
- comorbid conditions (such as hypertension, dyslipidaemia, hypogonadism, peripheral vascular disease, diabetes, obstructive sleep apnoea, obesity, liver, renal and thyroid disease);
- lower urinary tract symptoms;
- pelvic surgery, radiation or trauma;
- medications (Box 1); and
- psychiatric illness or conditions.
The use of validated sexual questionnaires such as the Sexual Health Inventory in Men (SHIM) and the International Index of Erectile Function (IIEF) scores18 can provide additional sexual information and be used as an indicator for treatment outcome.1,15
Physical examination
Assessment should include body habitus (including secondary sexual characteristics), peripheral vascular circulation, and neurological and genitourinary systems. Testicular examination is important to assess consistency (for atrophy or testis mass) and ensure that both testes are present. The identification of penile deformities may be best achieved in the erect state but is most examined by stretching the flaccid penis fully to palpate for Peyronie plaques.19
Laboratory testing
All major organisations recommend that laboratory tests should include fasting glucose level, lipid profile and, in selected cases, a hormone profile.1,4,8,9,10,11,12,13,14,15 It is advisable to screen for male hypogonadism in a man with ED and hypoactive desire, incomplete response to oral phosphodiesterase type 5 inhibitor (PDE5i) treatment, and with known type 2 diabetes and ED.20 Testosterone replacement therapy is indicated under the revised Pharmaceutical Benefits Scheme if the total testosterone threshold level is under 6 nmol/L on two separate readings (or testosterone between 6 nmol/L and 15 nmol/L with > 1.5 times the upper limit of luteinising hormone‐releasing hormone) in the absence of pituitary or testicular disease.21
Other tests such as thyroid‐stimulating hormone, luteinising hormone‐releasing hormone, sex hormone binding globulin, prolactin, full blood count, urea, creatinine and electrolytes, and urinalysis are considered complementary but are added when dictated by the clinical context, with subsequent referral for specialist care for further management if the results are abnormal.
Specialised testing
Most patients do not need further investigation unless specifically indicated, such as young age, known history of pelvic trauma, and in the setting of abnormality of the penis or testis on examination. Some patients who wish to know the aetiology of their ED, or for medico‐legal issues, can request further specialised testing following appropriate counselling.
Psychological/psychiatric assessment. Referral to a psychiatrist or psychologist with a special interest in sexual dysfunction can provide useful insight into the relationship and situational causes for ED. The goals of psychosexual therapy are to reduce or eliminate performance anxiety; to understand the context in which a man or a couple function sexually, including various interpersonal and psychosocial stressors; to implement appropriate psychosexual education to modify sexual scripts; and to improve treatment compliance.22 However, the lack of widespread availability and the additional cost and time factor can limit their use in most cases of ED treatment.
Diagnostic penile vascular imaging studies. Penile colour duplex ultrasound provides an inexpensive, simple and safe diagnostic evaluation of penile ultrastructures such as cavernosal smooth muscle, tunical plaque and vascular parameters.23 It should be performed by someone with experience in penile colour duplex ultrasound.20,23,24 and with concurrent use of intracavernosal vasoactive drug to provide adequate penile erection at the time of the study.24 A peak systolic velocity greater than 25 cm/s is considered normal post‐injection cavernosal arterial flow, and an end‐diastolic velocity of less than 5 cm/s excludes possible veno‐occlusive dysfunction (venous leak). If the penile colour duplex ultrasound is abnormal, penile arteriography or dynamic infusion cavernosometry and cavernosography can be performed, after appropriate patient counselling, to document the intracavernosal pressure and identify the specific location of a cavernous venous leak.25 Penile arteriography is an invasive test and should be reserved generally for cases of high flow priapism, or if patients are potential candidates for vascular reconstructive surgery.1,8,9,10,11,12,13,14,15
Neurophysiological testing. The nocturnal penile tumescence (NPT) test measures night‐time erectile events where a man should achieve about three to five penile erections per 8 hours of sleep.26 At present, there is a lack of consensus on acceptable NPT parameters to define true ED because the duration and intensity of nocturnal erections are likely related to the patient’s age, the environment of the NPT study, and the patient’s mental state on the day of the study.27 Furthermore, there are currently no NPT devices approved by the Therapeutic Goods Administration.
Other electrodiagnostic testing, such as bulbocavernosus reflex response, sympathetic skin response, pudendal somatosensory evoked responses, and penile biothesiometry, can provide an indirect measure of peripheral nervous system integrity but have limited clinical availability and utility, and hence these tests should be reserved for specific neurological conditions only.28
Summary recommendations
- A comprehensive clinical history and tailored physical examination are required in all cases (LoE 3; Grade B).
- Clinically validated questionnaires to evaluate ED can be used to assess sexual function domains and response to therapies (LoE 3; Grade B).
- Routine blood testings for ED include fasting glucose and/or glycated haemoglobin, lipid profile and fasting testosterone levels (LoE 3; Grade A).
- Specialised diagnostic tests are required under certain circumstances only, and proper patient counselling should be undertaken before organising these tests (LoE 4; Grade B).
Management strategies
The management of ED will most often occur concurrently with lifestyle modification and treatment of organic or psychosexual issues. Patients with risk factors for cardiovascular disease should be referred for further cardiac investigation or a cardiologist if indicated. Those at high risk of cardiovascular disease, especially if clinically manifesting angina, for example, should defer ED treatment until an appropriate cardiological assessment is undertaken (Expert opinion). Patients (and partners) should be made aware of clinical efficacy, risks and benefits of appropriate treatments, taking into consideration patient preferences and expectations. Stepwise progression from the least invasive oral agents through to second and third line therapies should occur as required (Box 2). Ongoing monitoring and support every 6–12 months, as required, should be provided by the clinician to maximise treatment success.
Established and recommended therapies
Oral phosphodiesterase type 5 inhibitor. Oral PDE5i medication is accepted as first line drug therapy, and all patients should be tried with PDE5i unless there is a contraindication (Box 3) (Expert opinion). The choice of initiating which PDE5i may be influenced by several factors, including timing or frequency of intercourse and interactions with food or alcohol.12,29 PDE5i therapy failure may be salvaged by patient re‐education about the timing of sexual activity and/or food consumption in relation to respective PDE5i. Other strategies include further optimisation of existing medical comorbidities and PDE5i dosing, including switching to a different PDE5i.12 In addition, testosterone replacement therapy should be offered to men with untreated male hypogonadism who are non‐responders to PDE5i drug.1,8,9,10,11,12,13,14,15
The prescription of PDE5i is contraindicated in men with unstable angina or are currently taking nitrates therapy, as life‐threatening hypotension may occur from excessive blood vessel relaxation.12,29 Each product description of PDE5i includes precautions on concomitant use with α‐blockers in men, and it is recommended that they take PDE5i at least 4–6 hours after the administration of the α‐blocker.30
Penile rehabilitation using PDE5i is considered the standard of care following radical prostatectomy and should be instituted early to protect and prevent corporal hypoxia and fibrosis.31 Even though there is no consensus on the exact timing, dose and duration of PDE5i therapy and its impact on non‐nerve‐sparing surgery and other forms of prostate cancer treatment modalities, regular use of PDE5i and appropriate escalation of pro‐erectile treatment strategies should be discussed in the context of prostate cancer survivorship.32
Intracavernosal injection (ICI) vasoactive agents and vacuum constriction device. ICI of vasoactive agents such as prostaglandin E1 (PGE1; also known as alprostadil) and combination agents (such as bimix = papaverine + phentolamine, or trimix = bimix + PGE1), which can be prescribed and administered by both general practitioners and urologists, is recommended as second line therapy in men who failed oral PDE5i (Box 3).1,12,15 Alprostadil is often prescribed as the initial ICI therapy, with the dose titrated based on the erectile responses, and can be co‐administered with PDE5i.1,12,15 Alprostadil is the only commercially mass‐produced “ready to use” ICI drug currently in Australia. The other drugs available can be used with/without alprostadil in their formulations, and as these are manufactured in an authorised and accredited compounding facility, the exact combinations of drugs and their respective dosages are dependent on the respective compounding pharmacies and may not be uniform between pharmacies (Expert opinion). Verapamil, vasoactive intestinal peptide, and forskolin can be additionally mixed with the main drugs, although the clinical data on these drugs are insufficient and should not be routinely used.12 The ICI therapy is often associated with low treatment compliance and high drop‐out rates due to several factors, such as desire for a permanent modality of therapy, fear of needles, poor response, fear of complications, and lack of sexual spontaneity.12,15 Priapism is a known complication of ICI therapy, especially in high doses, and can be associated with permanent penile damage.15 Hence, the prescription of these ICI drugs should include a detailed discussion with the patient, with technical instruction and education about priapism.
Vacuum constriction devices provide passive engorgement of the corpora cavernosa, via negative pressure, with a cylinder and attached pump, and a constriction ring can be placed at the base of the penis to maintain penile tumescence. Although vacuum constriction devices are highly effective in inducing erections, reported satisfaction rates can vary widely.1,12 Penile rigidity is achieved distal to the constriction band, and the base of the penile shaft proximal to the band will have some degrees of instability, resulting in pivoting at the base and the need for manual assistance to insert the erect penis for penetrative intercourse. Common adverse events include pain, inability to ejaculate, bruising and temporary numbness, while serious adverse events, such as skin necrosis, are uncommon if the use of the devices is limited to 30 minutes a day.33
Penile prosthesis implant. The penile prosthesis implant offers men a definitive treatment to restore erectile function and should be offered to patients who do not respond to, tolerate, or are unwilling to consider more conservative options. The inflatable penile prosthesis implant is considered a superior option to the malleable penile prosthesis because it produces penile rigidity and flaccidity that closely replicates a normal penile erectile function. While the penile prosthesis implant remains an effective, safe and durable treatment option for ED, it is irreversible, as subsequent removal of the penile prosthesis will not restore baseline erectile capability. Strict patient selection and counselling, stringent adherence to antimicrobial prophylaxis, and safe surgical practice are paramount to ensure low complication and high patient satisfaction rates.34 Scientific advances in penile prosthesis designs and materials coupled with better surgical techniques have made the penile prosthesis implant a more natural, durable and reliable device.35
Novel and emerging regenerative therapies
In contrast to the existing ED treatment regime, regenerative technology aspires to promote endothelial revascularisation and modulate the neurohormonal pathway with various angiogenic and tissue growth factors. The evidence supporting the use of low intensity extracorporeal shockwave therapy is accruing and recent guidelines have advocated this therapy to be safe and reasonably effective in mild to moderate ED, younger age groups, patients with minimal cardiovascular comorbidities, and in the absence of diabetes mellitus or cavernous nerve injury.36 However, the lack of standardised treatment protocols and different machines and energies used, coupled with the unknown longer term actual physiological changes in penile tissues, mean low intensity extracorporeal shockwave therapy should currently be offered in clinical trial settings following adequate patient counselling.37
While in principle the use of cellular‐based technology such as stem cell therapy and platelet‐rich plasma appears promising, safety risks with genomic or epigenetic changes in the longer term, as well as potential immune reactions and infection risks, need to be identified in more stringent clinical trials.38,39 Considering the lack of high level evidence in men with ED, there is a serious concern due to commercialisation and financial gain over patient wellbeing in this vulnerable patient demographic. For these reasons, there is a need for appropriate government regulation in local bioactive agents and regenerative cellular‐based therapy for ED.
Summary recommendations
- Lifestyle changes, management of risk factors and optimisation of existing medical conditions should accompany all ED treatment regimens (LoE 1; Grade A).
- Cardiovascular risk stratification should be performed, and referral for further cardiac testing and cardiology review should be provided (LoE 1; Grade A).
- Oral PDE5i drug remains the first line therapy for ED (LoE 1; Grade A).
- Correction of male hypogonadism may improve sexual function and salvage non‐responders to PDE5i (LoE 2; Grade A).
- ICIs and vacuum erection devices are recommended as second line therapy (LoE 1; Grade B).
- Penile prosthesis implantation can be considered in men who are medically refractory or unable to tolerate the side effects of medical therapy (LoE 4; Grade B).
- The use of low intensity shockwave therapy, pro‐erectile regenerative and cellular‐based therapy should be cautioned and remains largely experimental (LoE 3; Grade B).
Conclusion
ED is a common condition and patients with medical comorbidities should be thoroughly evaluated for cardiovascular and metabolic risk factors and managed accordingly. Furthermore, the current evidence supports a multidisciplinary approach with personalised medicine to tailor the management of ED. The availability of effective and safe PDE5i medication has led to an increase in the number of men seeking help for ED, thereby creating an opportunity to screen and promote good sexual health and safe sexual practice. More invasive second and third line treatments can be offered in cases where better erection is desired and/or failure of oral medication is encountered.
This set of clinical practice recommendations is intended to provide a clinical framework and serve as a guide in the decision making on the management of ED. The strategies and approaches recommended in these clinical guidelines are derived from evidence‐based and consensus‐based processes endorsed by USANZ and the AChSHM for the RACP. The most effective approach for a particular patient with ED is best determined by the patient (and his partner) and treating clinician in the context of that patient’s history, values and goals for treatment.
Box 1 – Common drugs that may contribute to erectile dysfunction
|
Class of drug |
Examples |
||||||||||||||
|
|
|||||||||||||||
|
Antihypertensives |
|
||||||||||||||
|
Diuretics |
|
||||||||||||||
|
Cardiac |
|
||||||||||||||
|
Antidepressants |
|
||||||||||||||
|
Hormones |
|
||||||||||||||
|
Antihistamines |
|
||||||||||||||
|
Anticholinergics |
|
||||||||||||||
|
Chemotherapy |
|
||||||||||||||
|
|
|||||||||||||||
|
LHRH = luteinising hormone‐releasing hormone; SNRI = serotonin–noradrenaline reuptake inhibitor; SSRI = selective serotonin reuptake inhibitor. |
|||||||||||||||
Box 2 – Algorithm for the management of erectile dysfunction
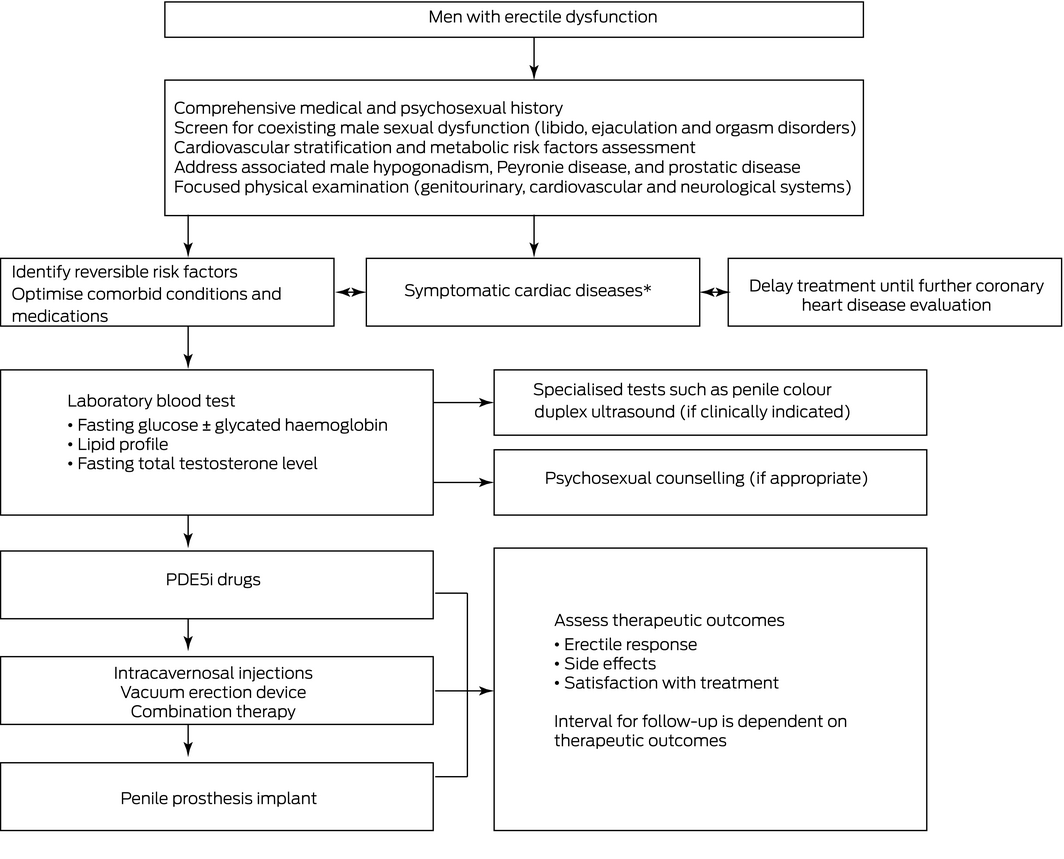
PDE5i = phosphodiesterase type 5 inhibitor. * Symptomatic cardiac diseases include history of unstable angina, recent myocardial infarction (< 6 months), poorly controlled congestive cardiac failure, significant cardiomyopathy and/or untreated cardiac arrhythmia.
Box 3 – Oral phosphodiesterase type 5 inhibitor (PDE5i) and intracavernosal injection (ICI) drugs currently available in Australia
|
Drugs |
Dose |
Median Tmax |
Half‐life |
Precaution |
Contraindications |
Side effects |
|||||||||
|
|
|||||||||||||||
|
Sildenafil |
25 mg PRN, 50 mg PRN and 100 mg PRN |
60 min |
4 h |
Consider decrease dose in older patients (aged > 65 years), hepatic or renal impairment, concomitant use of potent cytochrome P450 drugs |
Patients taking nitrate medications and those with known hypersensitivity to any component of the tablet |
|
|||||||||
|
Vardenafil |
10 mg PRN and 20 mg PRN |
60 min |
4 h |
||||||||||||
|
Tadalafil |
5 mg (daily dose), 10 mg PRN and 20 mg PRN |
120 min |
17.5 h |
||||||||||||
|
Avanafil |
50 mg PRN, 100 mg PRN, 200 mg PRN |
30 min |
6–17 h |
||||||||||||
|
Alprostadil |
5–20 μg PRN |
5 min |
5–10 min |
Careful in patients taking antiplatelet or anticoagulant medications and in patients with hepatic or renal impairment |
Patients with known hypersensitivity to any component of the drug |
|
|||||||||
|
Papaverine |
Up to 120 mg PRN (not as single agent, usually as bimix with phentolamine or trimix with phentolamine and alprostadil) |
10 min |
1.5–2 h |
||||||||||||
|
Phentolamine |
0.5–1 mg/mL PRN (not as single agent, usually as bimix with papaverine or trimix with phentolamine and alprostadil) |
30 min |
2 h |
||||||||||||
|
|
|||||||||||||||
|
PRN = on‐demand use; Tmax = time to maximum plasma concentration. |
|||||||||||||||
Provenance: Not commissioned; externally peer reviewed.
- Eric Chung1,2,3
- Michael Lowy4
- Michael Gillman5
- Chris Love6
- Darren Katz7
- Graham Neilsen8
- 1 Princess Alexandra Hospital, Brisbane, QLD
- 2 University of Queensland, Brisbane, QLD
- 3 AndroUrology Centre, Brisbane, QLD
- 4 Male Clinic, Sydney, NSW
- 5 Men’s Health Doctor, Brisbane, QLD
- 6 Urology South, Melbourne, VIC
- 7 Men’s Health Melbourne, Melbourne, VIC
- 8 Stonewall Medical Centre, Brisbane, QLD
No relevant disclosures.
- 1. Montorsi F, Adaikan G, Becher E, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex Med 2010; 7: 3572‐3588.
- 2. Lewis RW, Fugl‐Meyer KS, Bosch R, et al. Epidemiology/risk factors of sexual dysfunction. J Sex Med 2004; 1: 35‐39.
- 3. Vlachopoulos CV, Terentes‐Printzios DG, Ioakeimidis NK, et al. Prediction of cardiovascular events and all‐cause mortality with erectile dysfunction: a systematic review and meta‐analysis of cohort studies. Circ Cardiovasc Qual Outcomes 2013; 6: 99‐109.
- 4. Nehra A, Jackson G, Miner M, et al. The Princeton III consensus recommendations for the management of erectile dysfunction and cardiovascular disease. Mayo Clin Proc 2012; 87: 766‐778.
- 5. Jackson G, Nehra A, Miner M, et al. The assessment of vascular risk in men with erectile dysfunction: the role of the cardiologist and general physician. Int J Clin Pract 2013; 67: 1163‐1172.
- 6. Gacci M, Eardley I, Giuliano F, et al. Critical analysis of the relationship between sexual dysfunctions and lower urinary symptoms due to benign prostatic hyperplasia. Eur Urol 2011; 60: 809‐825.
- 7. McMahon CG. Current diagnosis and management of erectile dysfunction. Med J Aust 2019; 210: 469‐476. https://www.mja.com.au/journal/2019/210/10/current‐diagnosis‐and‐management‐erectile‐dysfunction
- 8. Hatzimouratidis K, Amar E, Eardley I, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol 2010; 57: 804‐814.
- 9. Bella AJ, Lee JC, Carrier S, et al. 2015 CUA Practice guidelines for erectile dysfunction. Can Urol Assoc J 2015; 9: 23‐29.
- 10. Ryu JK, Cho KS, Kim SJ, et al. Korean Society for Sexual Medicine and Andrology (KSSMA) guideline on erectile dysfunction. World J Mens Health 2013; 31: 83‐102.
- 11. American Urological Association. Erectile dysfunction: AUA guidelines (2018) [website]. https://www.auanet.org/guidelines/erectile‐dysfunction‐(ed)‐guideline (viewed Jan 2020).
- 12. Hatzimouratidis K, Salonia A, Adaikan G, et al. Pharmacotherapy for erectile dysfunction: recommendations from the Fourth International Consultation for Sexual Medicine (ICSM 2015). J Sex Med 2016; 13: 465‐488.
- 13. Hackett G, Kirby M, Wylie K, et al. British Society for Sexual Medicine guidelines on the management of erectile dysfunction in men. J Sex Med 2018; 15: 430‐457.
- 14. Kimoto Y, Nagao K, Sasaki H, et al. JSSM guidelines for erectile dysfunction. Int J Urol 2008; 15: 564‐576.
- 15. Mulhall JP, Giraldi A, Hackett, G et al. The 2018 revision to the process of care model for evaluation of erectile dysfunction. J Sex Med 2018; 15: 1280‐1292.
- 16. Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM Evidence Levels of Evidence (introductory document). Oxford Centre for Evidence‐Based Medicine, 2011. https://www.cebm.ox.ac.uk/resources/levels‐of‐evidence/levels‐of‐evidence‐introductory‐document (viewed Aug 2022).
- 17. Guyatt GH, Oxman AD, Kunz R, et al; GRADE Working Group. Going from evidence to recommendations. BMJ 2008; 336: 1049‐1051.
- 18. Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5‐year review of research and clinical experience. Int J Impot Res 2005; 17: 307‐319.
- 19. Ghanem HM, Salonia A, Martin‐Morales A. SOP: Physical examination and laboratory testing for men with erectile dysfunction. J Sex Med 2013; 10: 108‐110.
- 20. Meuleman EJH, Hatzichristou D, Rosen RC, Sadovsky R. Diagnostic tests for male erectile dysfunction revisited. Committee Consensus Report of the International Consultation in Sexual Medicine. J Sex Med 2010; 7: 2375‐2381.
- 21. Bu BY, Grossmann M, McLachlan RI, et al. Endocrine Society of Australia position statement on male hypogonadism (part 1): assessment and indications for testosterone therapy. Med J Aust 2016; 205: 173‐178. https://www.mja.com.au/journal/2016/205/4/endocrine‐society‐australia‐position‐statement‐male‐hypogonadism‐part‐1
- 22. Schmidt HM, Munder T, Gerger H, et al. Combination of psychological intervention and phosphodiesterase‐5 inhibitors for erectile dysfunction: a narrative review and meta‐analysis. J Sex Med 2014; 11: 1376‐1391.
- 23. Chung E, Yan H, De Young L, Brock GB. Penile Doppler sonographic and clinical characteristics in Peyronie’s disease and/or erectile dysfunction: an analysis of 1500 men with male sexual dysfunction. BJU Int 2012; 110: 1201‐1205.
- 24. Sikka SC, Hellstrom WJ, Brock G, Morales AM. Standardization of vascular assessment of erectile dysfunction: Standard operating procedures for duplex ultrasound. J Sex Med 2013; 10: 120‐129.
- 25. Glina S, Ghanem H. SOP: corpus cavernosum assessment (cavernosography/cavernosometry). J Sex Med 2013; 10: 111‐114.
- 26. Elhanbly S, Elkholy A. Nocturnal penile erections: the role of RigiScan in the diagnosis of vascular erectile dysfunction. J Sex Med 2012; 9: 3219‐3226.
- 27. Jannini EA, Granata AM, Hatzimouratidis K, Goldstein I. Use and abuse of RigiScan in the diagnosis of erectile dysfunction. J Sex Med 2009; 6: 1820‐1829.
- 28. Giuliano F, Rowland DL. Standard operating procedures for neurophysiologic assessment of male sexual dysfunction. J Sex Med 2013; 10: 1205‐1211.
- 29. Yuan J, Zhang R, Yang Z, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta‐analysis. Eur Urol 2013; 63: 902‐912.
- 30. Jannini EA, DeRogatis LR, Chung E, Brock GB. How to evaluate the efficacy of the phosphodiesterase type 5 inhibitors. J Sex Med 2012; 9: 26‐33.
- 31. Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J Sex Med 2013; 10 (Suppl): 102‐111.
- 32. Chung E, Gillman M. Prostate cancer survivorship: a review of current literature in erectile dysfunction and the concept of penile rehabilitation following prostate cancer therapy. Med J Aust 2014; 200: 582‐585. https://www.mja.com.au/journal/2014/200/10/prostate‐cancer‐survivorship‐review‐erectile‐dysfunction‐and‐penile
- 33. Porst H, Burnett A, Brock G, et al. SOP conservative (medical and mechanical) treatment of erectile dysfunction. J Sex Med 2013; 10: 130‐171.
- 34. Levine LA, Becher E, Bella A, et al. Penile prosthesis surgery: current recommendations from the International Consultation on Sexual Medicine. J Sex Med 2016; 13: 489‐518.
- 35. Chung E. Penile prosthesis implant: Scientific advances and technological innovations over the last four decades. Transl Androl Urol 2017; 6: 37‐45.
- 36. Chung E, Lee J, Liu CC, et al. Clinical practice guideline recommendation on the use of low intensity extracorporeal shock wave therapy and low intensity pulsed ultrasound shock wave therapy to treat erectile dysfunction: the Asia‐Pacific Society for Sexual Medicine position statement. World J Mens Health 2021; 39: 1‐8.
- 37. Chung E, Wang J. A state‐of‐art review of low intensity extracorporeal shock wave therapy and lithotripter machines for the treatment of erectile dysfunction. Expert Rev Med Device 2017; 14: 929‐934.
- 38. Chung E. Stem‐cell‐based therapy in the file of urology: A review of stem cell basic science, clinical applications and future directions in the treatment of various sexual and urinary conditions. Expert Opin Biol Ther 2015; 15: 1623‐1632.
- 39. Scott S, Roberts M, Chung E. Platelet‐rich plasma and treatment of erectile dysfunction: Critical review of literature and global trends in platelet‐rich plasma clinics. Sex Med Rev 2019; 7: 306‐312.





Abstract
Introduction: These clinical practice recommendations by the Urological Society of Australia and New Zealand (USANZ) and the Australasian Chapter of Sexual Health Medicine (AChSHM) for the Royal Australasian College of Physicians (RACP) provide evidence‐based clinical guidelines on the management of erectile dysfunction (ED) in Australia.
Main recommendations:
Changes in management as a result of these guidelines: Modification of lifestyle behaviour, management of reversible risk factors and optimisation of existing medical conditions remain pivotal, and existing standard ED therapies are often effective and safe following cardiovascular risk stratification. Caution should be exercised on the use of regenerative technology in ED due to unknown long term outcomes.