The known: People with dementia in residential aged care are frequently prescribed psychotropic medicines, but significant benefit from such treatment is often unlikely.
The new: On entering residential care, the GPs for 72% of people with dementia changed; 44% were attended by GPs previously unknown to them. Polypharmacy and psychotropic medicine initiation were more common for these people than for other aged care residents.
The implications: A change of regular GP when entering residential care is an important factor in psychotropic medicine initiation. Better organisation of GP care handover and facilitating continuity of care could prevent potentially inappropriate psychotropic prescribing for aged care residents.
Aged care systems around the world are under pressure because of ageing populations and the increasing prevalence of dementia. Systemic weaknesses have been widely recognised,1,2 and inappropriate medicine use was among the problems scrutinised by the Australian Royal Commission into the Quality and Safety of Aged Care, particularly the use of antipsychotics and sedatives as chemical restraints.2,3 Polypharmacy is common in residential aged care,4,5 as is potentially inappropriate prescribing.4,6 In Australian aged care facilities, psychotropic medicines (antipsychotics, benzodiazepines, antidepressants) are often dispensed to people with dementia,7 especially soon after entry into residential care, a critical transition point.8
Changed prescribing for people entering residential care may reflect events that precipitated their entry or their adjustment to their new surroundings.9 For example, antipsychotic medicines can be indicated for treating the behavioural and psychological symptoms of dementia, including hallucinations and agitation.3 However, given the risk of adverse events (including stroke and death),10 it is recommended that such treatment be of short duration3 and that non‐pharmacological approaches, such as behavioural management therapy, be preferred. Despite efforts to reduce prescribing of antipsychotics,11,12 rates remain high in residential aged care.8
One potential major adjustment for people during the transition to residential care is a change in general practitioner.13 GPs are the major prescribers in Australian residential aged care,14 but little is known about how many residents change GPs when they enter aged care facilities, or the effect this has on their care. For people with dementia, a new environment can be distressing, and the impact can be exacerbated by having an unfamiliar GP. Assuming care of a new patient with dementia can be difficult for GPs because of communication barriers and lack of familiarity with the patient and the reasons for their admission. The Royal Commission noted that restraining patients, including pharmacologically, arises from a “lack of knowing the person as an individual person.”2
The importance for high quality primary care of maintaining a continuous patient–GP relationship is widely recognised.15 However, the Australian Medical Association has recently highlighted concern among Australian GPs regarding their ability to support patients when they enter residential care.15 While the number of GP consultations in residential aged care has increased,16 more than one‐third of GPs have reported that they intended to reduce their number of visits.15
We explored GP continuity for people with dementia entering residential care and how it influences their medicine use, examining associations with both overall prescribing (including polypharmacy) and that of psychotropic medicines in particular.
Methods
Our retrospective data linkage study was part of the Exploring the relationship between Social care, primary and secondary Health service use and adverse health OUTcomes (SHOut) project. We analysed data from the Sax Institute 45 and Up Study17 for a prospective cohort of 267 153 New South Wales people aged 45 years or more, randomly selected from the Services Australia enrolment database and recruited during 1 January 2006 – 31 December 2009. Participants joined the study by completing a questionnaire and providing written consent to long term linkage of their data with administrative health datasets (online Supporting Information).
Residential aged care residents
We included 45 and Up participants with diagnoses of dementia who entered permanent residential aged care during 1 January 2010 ‒ 30 June 2014 and were alive six months after entry, who had been dispensed medications during the preceding two years only as concessional beneficiaries, and for whom at least three GP claims had been lodged prior to entry and at least one after entry into residential care. People with dementia were identified using previously described criteria:18 any claim for dementia‐specific medications (donepezil, rivastigmine, galantamine, memantine), or dementia diagnosis codes in hospitalisation records, aged care assessments, or the Aged Care Funding Instrument (used to assess required level of care) between July 2006 and entry into permanent residential care.
General practitioners
The category of GP most frequently seen by a resident during the six months after residential care entry was determined by comparing Medicare Benefits Schedule (MBS) claims for GP visits (Supporting Information, table 1) during this period with MBS records for the 24 months preceding entry. Three categories were defined: “usual” when the GP most frequently seen by a resident had also been their most frequent GP prior to entry; “known” when the resident had seen the GP prior to entry but the GP was not their usual GP; and “new” when the resident had not seen the GP prior to entry to residential care.
Medicines dispensed
We counted the number of unique medicines dispensed during the six months before and the six months after entry into residential care, by seven‐digit World Health Organization anatomical therapeutic classification (ATC)19 groupings (chemical substances). Cumulative polypharmacy was defined as five or more20 and cumulative hyperpolypharmacy as ten or more concomitantly prescribed medicines. Psychotropic medicines were grouped as in similar investigations:8 antipsychotics (ATC codes N05A*, excluding prochlorperazine and lithium), benzodiazepines (N05BA*, N05CD*, N05CF*, N03AE*), and antidepressants (N06A*). Initiation was defined as new dispensing of a drug to a resident who had not been prescribed the drug during the preceding two years.
Covariates
Socio‐demographic factors, self‐reported health conditions, risk factors, and other health care utilisation characteristics that might confound the relationship between GP category and medicine use were included as covariates (Supporting Information, table 2).
Statistical analyses
Characteristics of residents by GP category are summarised as descriptive statistics. Multinomial propensity scores were calculated by generalised boosted regression, including all measured covariates in the model. The inverse of the propensity score provides an inverse probability of treatment (IPT) weight for balancing covariates across groups.
IPT‐weighted mean numbers of medicines and proportions of residents subject to polypharmacy, hyperpolypharmacy, or psychotropic medicine dispensing were calculated. General linear models were fitted to assess differences between GP groups using the IPT weights and adjusted for prior emergency hospitalisation and number of medicines, poly‐ and hyperpolypharmacy, or medicine use, as appropriate.
Four sensitivity analyses were conducted to explore potential confounding: analyses restricted to people who received GP services in the same general geographic location (within 6 km) before and after entering residential care; separate analyses for people who with or without emergency hospitalisations during the 30 days preceding residential care entry; analyses restricted to medicines for which there were at least two claims within six months (to exclude “as required” prescribing); and analyses using residential care entry dates that incorporated prior respite residential care.
We conducted all weighted analyses in the survey package21 for R 4.0.2020 (R Project for Statistical Computing).
Ethics approval
The 45 and Up Study was approved by the University of New South Wales Human Research Ethics Committee (HC 15408). The SHOut study (including data linkage) was approved by the human research ethics committees of NSW Population and Health Services (HREC/15/CIPHS/57), the Aboriginal Health and Medical Research Council of NSW (1172/16), the Department of Veterans’ Affairs (E017/011), and the Australian Institute of Health and Welfare (EO2016/2/254).
Results
A total of 2250 residents with dementia were included in our study (Box 1). Their mean age was 84.1 years (standard deviation [SD], 7.0 years; 1236 were women (54.9%). The mean number of GP visits per participant during the two years preceding entry into residential care was 28 (SD, 17), the median number of providers was four (interquartile range [IQR], 3–6); in the six months after entry, they had a mean 12 visits (SD, 8) by a median two providers (IQR, 1–3). The most frequently seen GP in residential care was their usual GP for 625 residents (27.8%), a known GP for 645 residents (28.7%), or a new GP for 980 residents (43.6%).
The mean age of residents seeing new GPs was marginally lower than for the other two groups; larger proportions had annual household incomes below $20 000, lived in major cities, reported no current medical conditions, had seen a GP less than ten times during the year preceding entry into residential care, or had emergency hospitalisations shortly before entering residential care; their median number of dispensed medicines prior to residential care entry was slightly lower than for the other two groups. The proportions of residents who entered residential care needing higher levels of daily assistance or with more complex health care needs (Aged Care Funding Instrument) were slightly larger in the new GP group than in the other two groups (Box 2).
After applying IPT weighting, reasonable balance was achieved across groups (indicated by a standardised mean difference of less than 0.1), with the exception of prior emergency hospitalisation and number of hospital days (Supporting Information, table 4). As these two variables were highly correlated, only the variable with the greatest imbalance (prior emergency hospitalisation) was included in our models.
Numbers of medicines dispensed
The mean number of medicines dispensed to new residents increased by 1.1 medicines (95% confidence interval [CI], 0.9–1.3 medicines), from 8.2 (95% CI, 8.0–8.4) medicines during the six months preceding residential care to 9.3 (95% CI, 9.1–9.5) medicines during the first six months in residential care. The increase in mean number of medicines for the new GP group (+1.6 medicines; 95% CI, 1.4–1.9 medicines) was larger than for the usual GP group (+0.7 medicines; 95% CI, 0.4–1.1 medicines; adjusted rate ratio [aRR], 2.42; 95% CI, 1.59–3.70); the mean increases for the known (+0.8 medicines; 95% CI, 0.5–1.2 medicines) and usual GP groups were similar (aRR, 1.12; 95% CI, 0.71–1.75) (Box 3). The medication types most frequently initiated were other analgesics and antipyretics (N02B), drugs for constipation (A06A), opioids (N02A), and antipsychotics (N05A); the types most frequently discontinued were lipid‐modifying agents, plain (C10A) and other β‐lactam antibacterial drugs (J01D) (Supporting Information, table 5).
Polypharmacy
Cumulative polypharmacy was noted for 1729 participants (76.8%) before entering residential care, and for 1941 residents after entry (86.3%); hyperpolypharmacy was noted for 791 residents before (35.2%) and for 986 residents after entering residential care (43.8%). In residential care, polypharmacy (859 residents; weighted proportion, 88.6%) and hyperpolypharmacy (441 residents; weighted proportion, 46.2%) were more frequent in the new GP group than in the usual GP (530 residents, 84.6% and 251, 33.8% respectively) and known GP groups (552 residents, 84.6% and 294, 43.2% respectively). After weighting and adjusting for pre‐residential care levels of poly‐ and hyperpolypharmacy and for emergency hospitalisation, the odds of polypharmacy (adjusted odds ratio [aOR], 1.53; 95% CI, 1.09–2.14) and hyperpolypharmacy in residential care (aOR, 1.47; 95% CI, 1.14–1.89) were higher for the new GP group than for the usual GP group. Odds for the known and usual GP groups were similar (polypharmacy: aOR, 0.93; 95% CI, 0.64–1.36; hyperpolypharmacy: aOR, 1.21; 95% CI, 0.92–1.60).
Psychotropic medicines
Before entering residential care, 432 participants had been dispensed antipsychotics (19%), 408 benzodiazepines (18%), and 771 antidepressants (34%); after entry, 675 participants were dispensed antipsychotics (30%; median, four [IQR, 2–7] dispensings in six months), 513 benzodiazepines (23%; median, four [IQR, 1–7] dispensings), and 914 antidepressants (41%; median, six [IQR, 4–7] dispensings).
After weighting and adjusting for pre‐residential care levels of medicine use and prior emergency hospitalisation, the odds of being dispensed any psychotropic medicine (aOR, 1.64; 95% CI, 1.24–2.18), antipsychotics (aOR, 1.59; 95% CI, 1.18–2.12), or benzodiazepines (aOR, 1.69; 95% CI, 1.25–2.30) were each higher for the new GP than the usual GP group; those for the dispensing of antidepressants were similar (aOR, 1.32; 95% CI, 0.98–1.77). For all medicine types, the odds were similar for the usual and known GP groups (Box 4).
Similar differences between the new and usual GP groups were found in analyses restricted to residents who used GP services in the same general geographic location before and after entering residential care (Supporting Information, table 6), in separate analyses of residents who were or were not hospitalised during the 30 days preceding residential care (Supporting Information, table 7), in analyses restricted to medicines for which there had been at least two claims for a resident within six months (Supporting Information, table 8), and in analyses for which residential care entry dates were adjusted to include prior respite care (Supporting Information, table 9).
The odds of antipsychotics (aOR, 1.85; 95% CI, 1.31–2.61), benzodiazepines (aOR, 1.89; 95% CI, 1.24–2.90), and antidepressants (aOR, 1.64; 95% CI, 1.10–2.44) being initiated for residents were each higher for the new GP than the usual GP group. The odds of initiating antipsychotics (aOR, 1.31; 95% CI, 0.88–1.96), benzodiazepines (aOR, 1.27; 95% CI, 0.75–2.15), or antidepressants (aOR, 1.41; 95% CI, 0.89–2.21) were similar for the known GP and usual GP groups (Box 5).
Discussion
We found that most people with dementia changed GPs when they entered residential care: 44% to previously unfamiliar GPs, and 29% to GPs known to them but not their usual GPs. There are no national data with which to directly compare our estimates, but an earlier study in South Australia similarly found that 62‒76% of patients discharged from hospital to residential aged care facilities changed GPs.24 A recent Canadian study found that only 12% of new long term care residents saw their rostered family physician in the first six months after entry,25 a smaller proportion than in our study (28%).
Residents seeing new GPs were dispensed more medicines, including antipsychotics and benzodiazepines than other new residents with dementia, the increase in dispensing after entering residential care was greater for these people, and the proportion subject to polypharmacy was larger. New GPs may appropriately initiate new treatments in response to recent changes in a patient’s needs or a differing view of these needs. Polypharmacy in older people can be appropriate, but it also increases the risks of medication errors and hazardous interactions.26 The expected benefits of antipsychotics and benzodiazepines for older people with dementia are small and the risk of adverse effects is high, prompting recommendations to first try non‐pharmacological alternatives.3
In our study, most people in residential care seeing new GPs were from metropolitan areas (66%), and for a large proportion (38%) acute events (ie, emergency hospitalisations) had precipitated their entry into residential care. However, these factors did not account for differences in new medicine prescribing, suggesting that the transition to a new GP is an independent factor that influences increased prescribing in residential care. Moving to a new area for residential care inevitably entails a change in GP, but similar patterns of medicine dispensing were evident for residents treated by GPs in the same area before and after entering residential care.
Prescribing could be reduced by recognising the difficulties faced by GPs caring for new patients in residential care. Better support could be provided by promoting continuity of care or more structured handover of GP care, such as multidisciplinary care planning during residential care entry, including geriatrician and medication reviews. The Royal Commission into the Quality and Safety of Aged Care noted that remuneration for GPs attending people in residential care is unsatisfactory, providing no incentive to take the time to adequately assess patients.2 While it may be impractical for GPs to continue caring for all their patients who enter residential care, removing financial and administrative barriers, as highlighted by the Australian Medical Association,15 may assist continuity of care. However, proposals to promote GP specialisation and accreditation in aged care may further fragment GP care.27
Strengths and limitations
The major strengths of our study were the large sample size and our adjusting for a wide range of socio‐demographic and health factors. Our study was observational, and causal inferences can therefore not be drawn, but the use of IPT weighting strengthens the reliability of our conclusions.
We relied on administrative data for identifying people with dementia, GP care, and medicine use, but we have previously found that drawing on multiple administrative datasets detects most people with dementia in a population sample.18 We will have underestimated usual provider care by assessing MBS claims if a GP used more than one provider number for the treatment of single patients; this is unlikely, and, in any case, misclassification would probably reduce differences between groups. A pharmaceutical dispensing claim does not necessarily indicate that a dispensed medicine was taken. However, for most residents there were multiple claims, and our findings were not markedly affected by restricting analysis to drugs for which there were two or more claims, suggesting active use of the medicine. We had no information about clinical indications for prescribing, and it therefore was not possible to determine its appropriateness for individual residents. We limited our study to people who lived in residential care for at least six months to allow adequate assessment of GP patterns, but this limitation also avoided confounding by end‐of‐life prescribing patterns. Finally, we did not have access to residential aged care facility identifiers, and had only limited information about GP and care facility characteristics that influence prescribing patterns, such as the GP care model.
Conclusions
Medicine use increases to a greater degree and psychotropic drugs are dispensed at higher rates for people with dementia who change GP when they enter residential aged care than for people who continue seeing their regular GP. Facilitating GP continuity of care and better supporting GP handover processes could help prevent potentially inappropriate initiation of psychotropic medicines.
Box 1 – Selection of participants for inclusion in our retrospective data linkage study
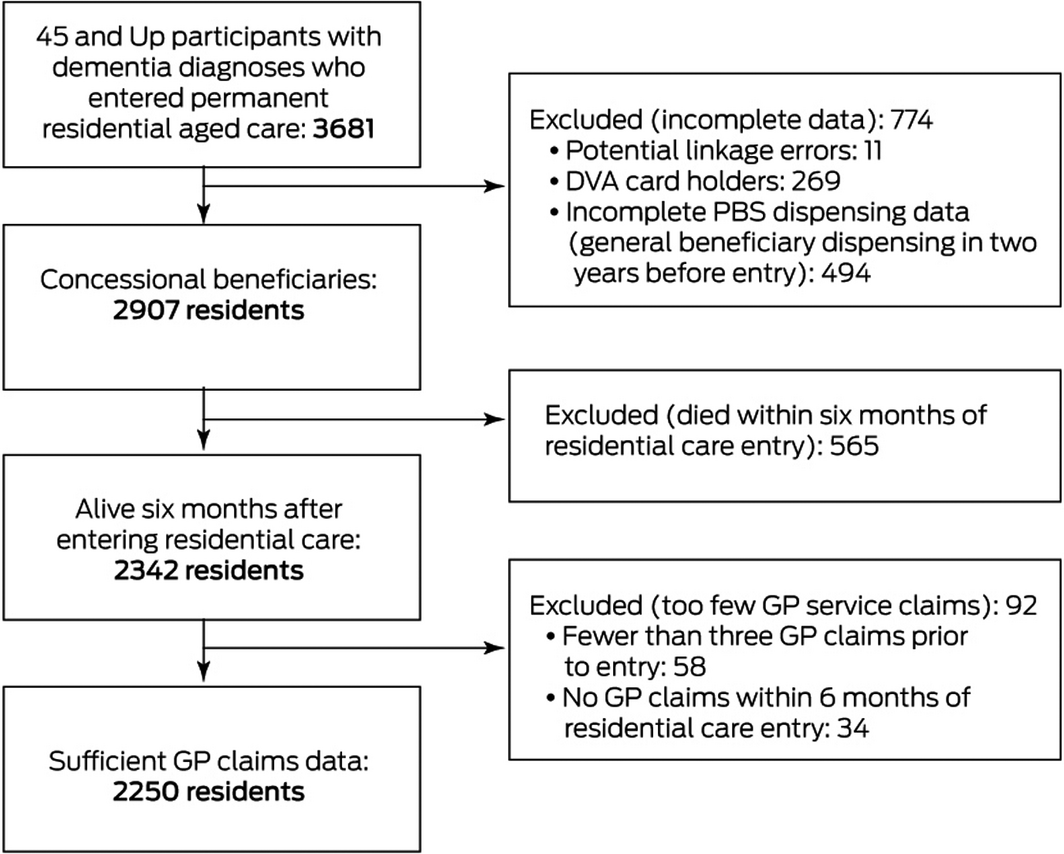
DVA = Department of Veteran’s Affairs; PBS = Pharmaceutical Benefits Scheme.
Box 2 – Characteristics of participants, by residential care general practitioner category
|
|
Most frequent GP seen |
||||||||||||||
|
Characteristic |
Usual GP |
Known GP |
New GP |
||||||||||||
|
|
|||||||||||||||
|
Number of residents |
625 |
645 |
980 |
||||||||||||
|
Age at entry (years), mean (SD) |
84.7 (7.0) |
84.1 (6.7) |
83.8 (7.2) |
||||||||||||
|
Sex (women) |
334 (53.4%) |
352 (54.6%) |
550 (56.1%) |
||||||||||||
|
Annual household income |
|
|
|
||||||||||||
|
< $20 000 |
248 (39.7%) |
219 (34.0%) |
420 (42.9%) |
||||||||||||
|
≥ $20 000 |
147 (23.5%) |
175 (27.1%) |
203 (20.7%) |
||||||||||||
|
Missing/invalid data |
230 (36.8%) |
251 (38.9%) |
357 (36.4%) |
||||||||||||
|
Remoteness area22 |
|
|
|
||||||||||||
|
Major cities |
306 (49.0%) |
405 (62.8%) |
643 (65.6%) |
||||||||||||
|
Inner regional |
242 (38.7%) |
186 (28.8%) |
257 (26.2%) |
||||||||||||
|
Outer regional/remote/very remote |
71 (11.4%) |
51 (7.9%) |
69 (7.0%) |
||||||||||||
|
Self‐reported conditions |
|
|
|
||||||||||||
|
None |
153 (24.5%) |
163 (25.3%) |
289 (29.5%) |
||||||||||||
|
One |
237 (37.9%) |
236 (36.6%) |
332 (33.9%) |
||||||||||||
|
Two |
155 (24.8%) |
181 (28.1%) |
228 (23.3%) |
||||||||||||
|
Three or more |
80 (13%) |
65 (10%) |
131 (13.4%) |
||||||||||||
|
Self‐reported memory |
|
|
|
||||||||||||
|
Excellent |
21 (3.4%) |
33 (5.1%) |
48 (4.9%) |
||||||||||||
|
Very good |
71 (11.4%) |
77 (11.9%) |
123 (12.6%) |
||||||||||||
|
Good |
180 (28.8%) |
193 (29.9%) |
309 (31.5%) |
||||||||||||
|
Fair |
213 (34.1%) |
211 (32.7%) |
314 (32.0%) |
||||||||||||
|
Poor |
91 (14.6%) |
86 (13.3%) |
104 (10.6%) |
||||||||||||
|
Missing/invalid data |
49 (7.8%) |
45 (7.0%) |
82 (8.4%) |
||||||||||||
|
Physical limitations |
|
|
|
||||||||||||
|
No limitations |
47 (7.5%) |
63 (9.8%) |
88 (9.0%) |
||||||||||||
|
Minor limitation |
73 (11.7%) |
68 (10.5%) |
122 (12.4%) |
||||||||||||
|
Moderate limitation |
153 (24.5%) |
164 (25.4%) |
247 (25.2%) |
||||||||||||
|
Severe limitation |
234 (37.4%) |
211 (32.7%) |
323 (33.0%) |
||||||||||||
|
Missing/invalid data |
118 (18.9%) |
139 (21.6%) |
200 (20.4%) |
||||||||||||
|
Psychological distress |
|
|
|
||||||||||||
|
Low |
427 (68.3%) |
445 (69.0%) |
666 (68.0%) |
||||||||||||
|
Moderate |
95 (15%) |
86 (13%) |
146 (14.9%) |
||||||||||||
|
High |
34 (5.4%) |
39 (6.0%) |
57 (5.8%) |
||||||||||||
|
Very high |
14 (2.2%) |
18 (2.8%) |
24 (2.4%) |
||||||||||||
|
Missing/invalid data |
55 (8.8%) |
57 (8.8%) |
87 (8.9%) |
||||||||||||
|
Year preceding residential care |
|||||||||||||||
|
GP visits |
|
|
|
||||||||||||
|
< 10 |
219 (35.0%) |
169 (26.2%) |
398 (40.6%) |
||||||||||||
|
10–18 |
234 (37.4%) |
262 (40.6%) |
363 (37.0%) |
||||||||||||
|
19 or more |
172 (27.5%) |
214 (33.2%) |
219 (22.3%) |
||||||||||||
|
Specialist visits |
|
|
|
||||||||||||
|
None |
183 (29.3%) |
131 (20.3%) |
232 (23.7%) |
||||||||||||
|
One or two |
136 (21.8%) |
190 (29.5%) |
234 (23.9%) |
||||||||||||
|
Three or more |
306 (49.0%) |
324 (50.2%) |
514 (52.4%) |
||||||||||||
|
Time in hospital (weeks) |
|
|
|
||||||||||||
|
< 1 |
252 (40.3%) |
298 (46.2%) |
335 (34.2%) |
||||||||||||
|
1–4 |
178 (28.5%) |
179 (27.8%) |
243 (24.8%) |
||||||||||||
|
> 4 |
195 (31.2%) |
168 (26.0%) |
402 (41.0%) |
||||||||||||
|
Emergency department visits |
|
|
|
||||||||||||
|
None |
213 (34.1%) |
205 (31.8%) |
261 (26.6%) |
||||||||||||
|
One |
169 (27.0%) |
175 (27.1%) |
284 (29.0%) |
||||||||||||
|
Two or more |
243 (38.9%) |
265 (41.1%) |
435 (44.4%) |
||||||||||||
|
Emergency hospitalisation immediately before entering residential care |
168 (26.9%) |
114 (17.7%) |
375 (38.3%) |
||||||||||||
|
Highest level of home‐based aged care service |
|
|
|
||||||||||||
|
High level |
55 (8.8%) |
53 (8.2%) |
68 (6.9%) |
||||||||||||
|
Low level |
161 (25.8%) |
161 (25.0%) |
199 (20.3%) |
||||||||||||
|
Home support |
255 (40.8%) |
286 (44.3%) |
428 (43.7%) |
||||||||||||
|
No services |
154 (24.6%) |
145 (22.5%) |
285 (29.1%) |
||||||||||||
|
Level of care required at entry to residential care* |
|
|
|
||||||||||||
|
Activities of Daily Living |
|
|
|
||||||||||||
|
None or low |
255 (40.8%) |
300 (46.5%) |
354 (36.1%) |
||||||||||||
|
Moderate |
199 (31.8%) |
196 (30.4%) |
324 (33.1%) |
||||||||||||
|
High |
171 (27.4%) |
149 (23.1%) |
302 (30.8%) |
||||||||||||
|
Behaviour |
|
|
|
||||||||||||
|
None or low |
224 (35.8%) |
232 (36.0%) |
304 (31.0%) |
||||||||||||
|
Moderate |
158 (25.3%) |
158 (24.5%) |
249 (25.4%) |
||||||||||||
|
High |
243 (38.9%) |
255 (39.5%) |
427 (43.6%) |
||||||||||||
|
Complex health care |
|
|
|
||||||||||||
|
None or low |
385 (61.6%) |
412 (63.9%) |
560 (57.1%) |
||||||||||||
|
Moderate |
154 (24.6%) |
148 (22.9%) |
246 (25.1%) |
||||||||||||
|
High |
86 (14%) |
85 (13%) |
174 (17.8%) |
||||||||||||
|
Medicines in year preceding residential care, median number (IQR) |
10 (6.0–13) |
10 (7.0–15) |
9.0 (6.0–13) |
||||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range; SD = standard deviation. A more extensive list of characteristics by GP group is included in the Supporting Information, table 3. * Level of care required is categorised according to the Aged Care Funding Instrument matrix for each of three domains: activities of daily living, behaviour, and complex health care.23 We combined the no and low care categories in each domain because their numbers were small. |
|||||||||||||||
Box 3 – Numbers of medicines dispensed per person before and after entry into residential aged care, by residential care general practitioner category*
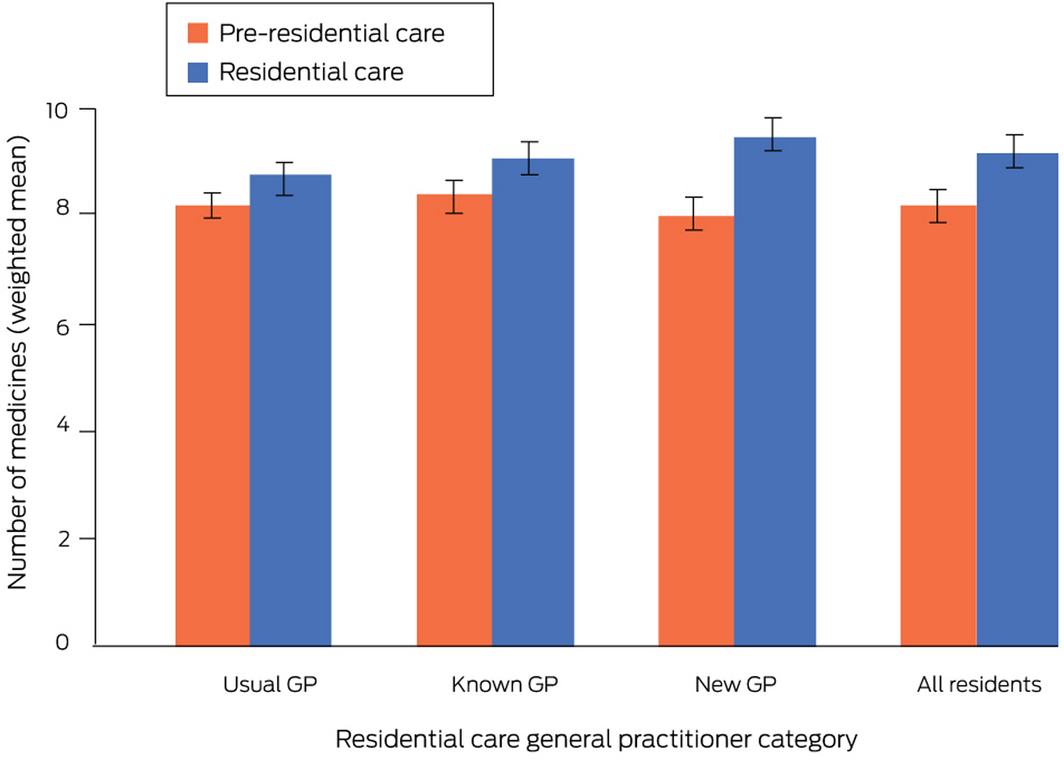
* Mean values are inverse probability of treatment‐weighted to balance the differences in covariates between general practitioner categories (Supporting Information, table 3).
Box 4 – Psychotropic medication dispensing before and after entry to residential care, by residential care general practitioner category
|
|
6 months before residential care entry |
6 months after residential care entry |
|
||||||||||||
|
Medicine type/GP category |
Number of residents |
Weighted proportion (95% CI) |
Number of residents |
Weighted proportion (95% CI) |
Adjusted weighted odds ratio (95% CI)* |
||||||||||
|
|
|||||||||||||||
|
Any psychotropic medicine |
|
|
|
|
|
||||||||||
|
Usual |
332 |
53.1% (48.9–57.3%) |
370 |
58.4% (54.3–62.6%) |
1 |
||||||||||
|
Known |
354 |
52.5% (48.3–56.8%) |
397 |
60.0% (55.9–64.2%) |
1.15 (0.85–1.56) |
||||||||||
|
New |
479 |
50.9% (47.6–54.2%) |
628 |
64.6% (61.4–67.7%) |
1.64 (1.24–2.18) |
||||||||||
|
Antipsychotics |
|
|
|
|
|
||||||||||
|
Usual |
120 |
19.2% (16.0–22.5%) |
163 |
26.4% (22.7–30.1%) |
1 |
||||||||||
|
Known |
150 |
22.8% (19.3–26.3%) |
199 |
30.8% (26.9–34.6%) |
1.18 (0.85–1.63) |
||||||||||
|
New |
162 |
16.4% (13.9–18.8%) |
313 |
30.8% (27.8–33.9%) |
1.59 (1.18–2.12) |
||||||||||
|
Benzodiazepines |
|
|
|
|
|
||||||||||
|
Usual |
123 |
19.3% (16.0–22.6%) |
128 |
19.2% (16.0–22.4%) |
1 |
||||||||||
|
Known |
131 |
17.9% (14.8–21.0%) |
143 |
21.7% (18.2–25.2%) |
1.32 (0.95–1.85) |
||||||||||
|
New |
154 |
16.4% (13.9–19.0%) |
242 |
24.5% (21.7–27.4%) |
1.69 (1.25–2.30) |
||||||||||
|
Antidepressants |
|
|
|
|
|
||||||||||
|
Usual |
219 |
34.8% (30.8–38.8%) |
245 |
38.6% (34.6–42.7%) |
1 |
||||||||||
|
Known |
225 |
32.8% (28.9–36.7%) |
262 |
39.0% (34.9–43.1%) |
1.15 (0.83–1.58) |
||||||||||
|
New |
327 |
35.3% (32.1–38.6%) |
407 |
42.7% (39.4–46.0%) |
1.32 (0.98–1.77) |
||||||||||
|
|
|||||||||||||||
|
CI = confidence interval. * Inverse probability of treatment‐weighted to balance differences in covariates between general practitioner categories (Supporting Information, table 3), and directly adjusted for prior dispensing and emergency hospitalisation. |
|||||||||||||||
Box 5 – Proportions of residents for whom psychotropic medicines were initiated within six months of entering residential aged care, by residential care general practitioner category*
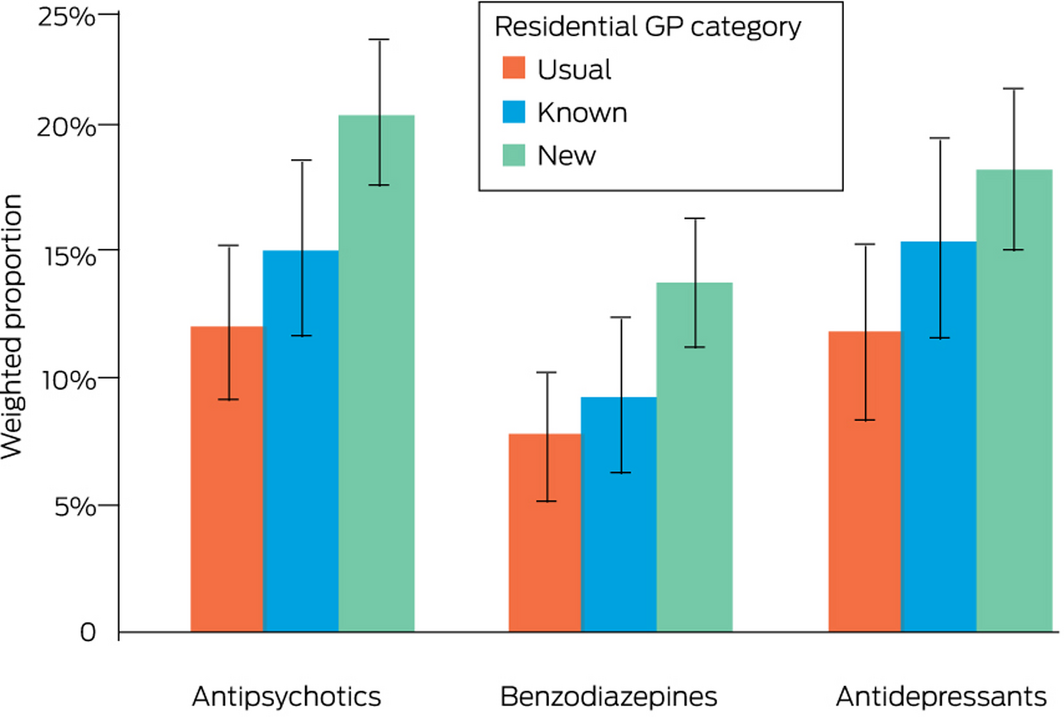
* At least one instance of dispensing to a resident for whom the medication type had not been prescribed during the two years preceding entry into residential care; proportions weighted by inverse probability of treatment based on differences in covariates between general practitioner categories (Supporting Information, table 3).
Received 11 December 2020, accepted 11 May 2021
- Heidi J Welberry1
- Louisa R Jorm1
- Andrea L Schaffer1
- Sebastiano Barbieri1
- Benjumin Hsu1
- Mark F Harris2
- John Hall3
- Henry Brodaty4,5
- 1 Centre for Big Data Research in Health, University of New South Wales, Sydney, NSW
- 2 Centre for Primary Health Care and Equity, University of New South Wales, Sydney, NSW
- 3 University of New South Wales, Sydney, NSW
- 4 Dementia Centre for Research Collaboration, University of New South Wales, Sydney, NSW
- 5 Centre for Healthy Brain Ageing, University of New South Wales, Sydney, NSW
Heidi Welberry was supported by an Australian Government Research Training Program scholarship and a PhD top‐up through the Maintain Your Brain NHMRC Boosting Dementia Research program (1095097).
No relevant disclosures.
- 1. Macdonald A, Cooper B. Long‐term care and dementia services: an impending crisis. Age Ageing 2007; 36: 16–22.
- 2. Royal Commission into Aged Care Quality and Safety. Restrictive practices. In: Interim report: Neglect, volume 1. Canberra: Commonwealth of Australia, 2019. https://agedcare.royalcommission.gov.au/sites/default/files/2020-02/interim-report-volume-1.pdf (viewed Apr 2020).
- 3. Royal Australian and New Zealand College of Psychiatrists. Antipsychotic medications as a treatment of behavioural and psychological symptoms of dementia (Professional Practice Guideline 10). Aug 2016. https://www.ranzcp.org/files/resources/college_statements/practice_guidelines/pg10-pdf.aspx (viewed Apr 2020).
- 4. Jokanovic N, Tan ECK, Dooley MJ, et al. Why is polypharmacy increasing in aged care facilities? The views of Australian health care professionals. J Eval Clin Pract 2016; 22: 677–682.
- 5. Onder G, Liperoti R, Fialova D, et al; SHELTER Project. Polypharmacy in nursing home in Europe: results from the SHELTER study. J Gerontol A Biol Sci Med Sci 2012; 67: 698–704.
- 6. Harrison SL, Kouladjian O’Donnell L, Bradley CE, et al. Associations between the drug burden index, potentially inappropriate medications and quality of life in residential aged care. Drugs Aging 2018; 35: 83–91.
- 7. Smeets CHW, Gerritsen DL, Zuidema SU, et al. Psychotropic drug prescription for nursing home residents with dementia: prevalence and associations with non‐resident‐related factors. Aging Ment Health 2018; 22: 1239–1246.
- 8. Harrison SL, Sluggett JK, Lang C, et al. The dispensing of psychotropic medicines to older people before and after they enter residential aged care. Med J Aust 2020; 212: 309–313. https://www.mja.com.au/journal/2020/212/7/dispensing-psychotropic-medicines-older-people-and-after-they-enter-residential
- 9. Toot S, Swinson T, Devine M, et al. Causes of nursing home placement for older people with dementia: a systematic review and meta‐analysis. Int Psychogeriatrics 2017; 29: 195–208.
- 10. Tampi RR, Tampi DJ, Balachandran S, Srinivasan S. Antipsychotic use in dementia: a systematic review of benefits and risks from meta‐analyses. Ther Adv Chronic Dis 2016; 7: 229–245.
- 11. Harrison F, Cations M, Jessop T, et al. Prolonged use of antipsychotic medications in long‐term aged care in Australia: a snapshot from the HALT project. Int Psychogeriatrics 2020; 32: 335–345.
- 12. Westbury JL, Gee P, Ling T, et al. RedUSe: Reducing antipsychotic and benzodiazepine prescribing in residential aged care facilities. Med J Aust 2018; 208: 398–403. https://www.mja.com.au/journal/2018/208/9/reduse-reducing-antipsychotic-and-benzodiazepine-prescribing-residential-aged
- 13. Reed RL. Models of general practitioner services in residential aged care facilities. Aust Fam Physician 2015; 44: 176–179.
- 14. Australian Institute of Health and Welfare. Mental health services in Australia. Updated Jan 2020. https://www.aihw.gov.au/reports/mental-health-services/mental-health-services-in-australia/report-contents/mental-health-related-prescriptions/prescriptions (viewed Apr 2020).
- 15. Australian Medical Association. 2017 AMA aged care survey report. July 2018. https://ama.com.au/article/2017-ama-aged-care-survey (viewed Apr 2020).
- 16. Australian Institute of Health and Welfare. Interfaces between the aged care and health systems in Australia: GP use by people living in permanent residential aged care 2012–13 to 2016–17 (Cat. no. AGE 103). Sept 2020. https://www.aihw.gov.au/getmedia/db38699f-86ec-44c1-84b0-96787b3805f6/aihw-age-103.pdf.aspx?inline=true (viewed Mar 2021).
- 17. 45 and Up Study Collaborators; Banks E, Redman S, Jorm L, et al. Cohort profile: the 45 and up study. Int J Epidemiol 2008; 37: 941–947.
- 18. Welberry HJ, Brodaty H, Hsu B, et al. Measuring dementia incidence within a cohort of 267 153 older Australians using routinely collected linked administrative data. Sci Rep 2020; 10: 8781.
- 19. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index 2020. https://www.whocc.no/atc_ddd_index (viewed Oct 2020).
- 20. Fincke BG, Snyder K, Cantillon C, et al. Three complementary definitions of polypharmacy: methods, application and comparison of findings in a large prescription database. Pharmacoepidemiol Drug Saf 2005; 14: 121–128.
- 21. Lumley T. Package “survey”: analysis of complex survey samples. Apr 2020. https://cran.r-project.org/web/packages/survey/survey.pdf (viewed Apr 2020).
- 22. Australian Bureau of Statistics. 1270.0.55.005. Australian Statistical Geography Standard (ASGS). Volume 5: remoteness structure, July 2011. Jan 2013. https://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/17A7A350F48DE42ACA258251000C8CA0?opendocument (viewed Dec 2020).
- 23. Australian Department of Health. Aged Care Funding Instrument (ACFI): user guide. 2016. https://www.health.gov.au/sites/default/files/documents/2020/01/aged-care-funding-instrument-acfi-user-guide-acfi-user-guide-2017.pdf (viewed Dec 2020).
- 24. Crotty M, Rowett D, Spurling L, et al. Does the addition of a pharmacist transition coordinator improve evidence‐based medication management and health outcomes in older adults moving from the hospital to a long‐term care facility? Results of a randomized, controlled trial. Am J Geriatr Pharmacother 2004; 2: 257–264.
- 25. Staykov E, Qureshi D, Scott M, et al. Do patients retain their family physicians after long‐term care entry? A retrospective cohort study. J Am Med Dir Assoc 2020; 21: 1951–1957.
- 26. Nguyen JK, Fouts MM, Kotabe SE, Lo E. Polypharmacy as a risk factor for adverse drug reactions in geriatric nursing home residents. Am J Geriatr Pharmacother 2006; 4: 36–41.
- 27. Australian Medical Association. AMA submission to the Royal Commission into Aged Care Quality and Safety: final recommendations by the counsel assisting. 17 Nov 2020. https://ama.com.au/articles/ama-submission-aged-care-royal-commission-response-counsel-assistings-recommendations (viewed Dec 2020).





Abstract
Objective: To examine relationships between changing general practitioner after entering residential aged care and overall medicines prescribing (including polypharmacy) and that of psychotropic medicines in particular.
Design: Retrospective data linkage study.
Setting, participants: 45 and Up Study participants in New South Wales with dementia who were PBS concession card holders and entered permanent residential aged care during January 2010 ‒ June 2014 and were alive six months after entry.
Main outcome measures: Inverse probability of treatment‐weighted numbers of medicines dispensed to residents and proportions of residents dispensed antipsychotics, benzodiazepines, and antidepressants in the six months after residential care entry, by most frequent residential care GP category: usual (same as during two years preceding entry), known (another GP, but known to the resident), or new GP.
Results: Of 2250 new residents with dementia (mean age, 84.1 years; SD, 7.0 years; 1236 women [55%]), 625 most frequently saw their usual GPs (28%), 645 saw known GPs (29%), and 980 saw new GPs (44%). The increase in mean number of dispensed medicines after residential care entry was larger for residents with new GPs (+1.6 medicines; 95% CI, 1.4‒1.9 medicines) than for those attended by their usual GPs (+0.7 medicines; 95% CI, 0.4‒1.1 medicines; adjusted rate ratio, 2.42; 95% CI, 1.59‒3.70). The odds of being dispensed antipsychotics (adjusted odds ratio [aOR], 1.59; 95% CI, 1.18‒2.12) or benzodiazepines (aOR, 1.69; 95% CI, 1.25‒2.30), but not antidepressants (aOR, 1.32; 95% CI, 0.98‒1.77), were also higher for the new GP group. Differences between the known and usual GP groups were not statistically significant.
Conclusions: Increases in medicine use and rates of psychotropic dispensing were higher for people with dementia who changed GP when they entered residential care. Facilitating continuity of GP care for new residents and more structured transfer of GP care may prevent potentially inappropriate initiation of psychotropic medicines.