The known: People with colorectal adenomas are at increased risk of cancer development, but the optimal surveillance interval after removing non‐advanced adenomas is not clear. Australian guidelines now recommend surveillance at ten rather than five years, but evidence for this change is limited.
The new: The estimated incidence of advanced neoplasia at the follow‐up colonoscopy was 19% at five years and 30% ten years after removal of a non‐advanced adenoma.
The implications: A longer surveillance interval after non‐advanced adenoma removal may entail an unacceptable level of risk of advanced neoplasia, particularly in older patients and those with a prior history of adenoma.
Australia has one of the highest rates of colorectal cancer in the world.1 Early detection by population screening — using colonoscopy, flexible sigmoidoscopy, or faecal occult blood tests, together with surveillance colonoscopy for people at higher risk2,3 — can reduce the risk of death.4
Detecting and removing precursor lesions (adenomatous and sessile serrated lesions) halt their development, averting the potential sequelae of colorectal cancer.5,6 National guidelines therefore recommend regular colonoscopy as a preventive strategy for people with these precursor lesions.7 Recommendations for surveillance following polyp removal are based on lesion characteristics predictive of future colorectal cancer. Based on observational studies, adenomas are classified as non‐advanced/low risk (one or two lesions, small [< 10 mm], low grade dysplasia) or as advanced/high risk (three or more lesions, large [≥ 10 mm], high grade dysplasia or villous change).8,9
Until recently, the Australian guidelines recommended surveillance colonoscopy five years after a finding of non‐advanced adenoma,10 but follow‐up at ten years is now recommended for most patients.7 While this is similar to the United States Multi‐Society Task Force guidelines11 and more conservative than the European Society of Gastrointestinal Endoscopy guidelines,12 the evidence supporting the change in timing is limited.
The recommended time interval between colonoscopies is related to the acceptable risk level of advanced neoplasia (colorectal cancer or advanced adenoma). A meta‐analysis found that the risk of advanced neoplasia at surveillance five years after removal of a non‐advanced adenoma was 4.9%, but the authors’ definition of advanced neoplasia did not include colorectal cancer.13 Others have suggested that advanced neoplasia yields of 10%14 and 15%15 would justify surveillance. Despite recognition of the importance of setting a surveillance threshold, a guidelines consensus has not been reached.
Given the potential consequences of delaying surveillance recall until ten years after detection of a non‐advanced adenoma, we investigated the incidence of advanced neoplasia in Australians undergoing surveillance after such findings. We also examined factors associated with the development of advanced neoplasia, including the timing of the surveillance colonoscopy.
Methods
We undertook a retrospective review of surveillance colonoscopy outcomes for patients in whom non‐advanced/low risk adenoma had previously been identified. We extracted data from the clinical database associated with the Southern Cooperative Program for the Prevention of Colorectal Cancer, an Adelaide program that coordinates surveillance colonoscopies for people at elevated risk of colorectal cancer at the Flinders Medical Centre, the Repatriation General Hospital, and Noarlunga Hospital.16
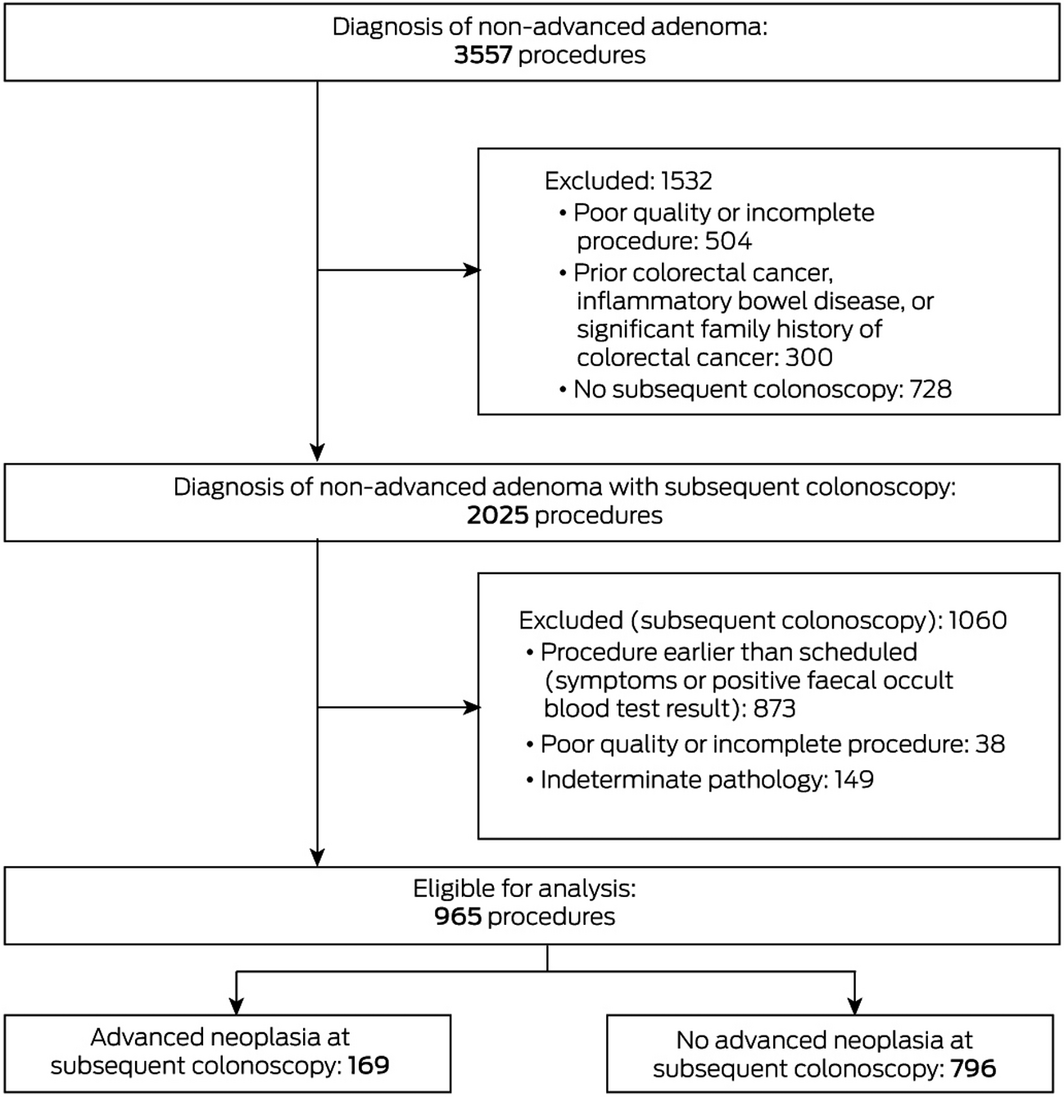
We extracted outcomes data for people who underwent colonoscopy during January 1999 – November 2016, including the quality of procedure and pathology. If non‐advanced adenoma was identified, we reviewed subsequent colonoscopies for findings of advanced neoplasia. Exclusion criteria included incomplete colonoscopy (caecum not reached or incomplete polyp resection), poor bowel preparation, a family history of colorectal cancer (conferring increased or high risk status, according to Australian guidelines17), prior resection of colorectal cancer, or a history of inflammatory bowel disease. Any subsequent colonoscopy performed earlier than scheduled (within 4.5 years of the preceding colonoscopy) because of gastrointestinal symptoms or positive faecal occult blood test results was also excluded, as the first procedure may have been inadequate in these cases.
We determined the influence of pathology, demographic characteristics, and surveillance interval length on detection of advanced neoplasia at the subsequent colonoscopy. Patient demographic characteristics (age, sex) were collected for the baseline procedure, as were the location and number of adenomas identified (one or two), largest size of non‐advanced adenoma (< 5 mm or 5–9 mm), number of previous colonoscopies, and time until subsequent surveillance colonoscopy.
To reflect Australian guidelines current during most of our study, advanced neoplasia was defined as colorectal cancer, advanced adenoma (at least one of: conventional adenoma ≥ 10 mm, villous change, high grade dysplasia, or three or more lesions), or advanced serrated polyps (traditional serrated adenomas of any size, sessile serrated lesions ≥ 10 mm, dysplasia, or three or more lesions).10 The diagnosis of sessile serrated lesions followed the 2010 World Health Organization guidelines;18 that is, diagnostic histologic features were present in at least three crypts or in two adjacent crypts. To conform with overseas classifications of advanced neoplasia, we also undertook a second analysis, excluding cases in which classification as advanced neoplasia was based on multiple small conventional adenomas or multiple small sessile serrated lesions without dysplasia.
Statistical analysis
D’Agostino–Pearson omnibus normality testing indicated that our continuous data were not normally distributed, and they are therefore summarised as medians with interquartile ranges (IQRs) or frequencies. Baseline comparisons between groups (with or without advanced neoplasia) were undertaken in Mann–Whitney U tests or χ2 tests. Risk factors for advanced neoplasia after non‐advanced adenoma were determined in multivariable logistic regression analyses adjusted for age, sex, history of adenoma, number of adenomas detected at baseline event, and time until surveillance colonoscopy. Model fit was evaluated with the Pearson goodness‐of‐fit statistic. Results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). Data were graphed to model the relationship between the incidence of advanced neoplasia and time until surveillance colonoscopy. As it has been suggested that an advanced neoplasia yield of 10%14 or 15%15 justifies surveillance, we also estimated times until these thresholds were reached. P < 0.05 was deemed statistically significant. Analyses were undertaken in Stata 16.0.
Ethics approval
Our study was approved by the Southern Adelaide Clinical Human Research Ethics Committee (reference, 93.19).
Results
Non‐advanced adenoma was the most significant finding in 3557 colonoscopies, including 2025 followed by surveillance colonoscopies; after exclusions, we included 965 index procedures in our analysis (Box 1). There were 904 unique patients (median age, 62.0 years; IQR, 54.0–69.0 years), of whom 570 were men (59.1%). The median interval between the initial and surveillance procedures was 5.2 years (IQR, 4.4–6.0 years; range, 2.0–14 years); 165 surveillance procedures were undertaken 2.0–3.9 years after the polypectomy (17.1%), 553 after 4.0–5.9 years (57.3%), 222 after 6.0–7.9 years (23.0%), and 25 after eight or more years (2.6%). Advanced neoplasia was identified in 169 surveillance colonoscopies, including one cancer (Box 2).
Risk factors associated with advanced neoplasia at surveillance colonoscopy
The median age at baseline was higher for patients with advanced neoplasia at the surveillance colonoscopy (64.0 years; IQR, 56.5–72.0 years) than for patients without advanced neoplasia (61.0 years; IQR, 53.0–68.0 years). The proportions by sex and the numbers of previous procedures were similar for the two groups (Box 2).
Neither the location nor the size of non‐advanced adenoma influenced the incidence of future advanced neoplasia. However, advanced neoplasia was more frequently identified at surveillance following initial colonoscopies in which two adenomas rather than one were found (26% v 16%). Advanced neoplasia was more frequently identified if adenoma had also been found during earlier colonoscopies (23% v 16%), regardless of type (Box 2).
Influence of surveillance interval on incidence of advanced neoplasia
The incidence of advanced neoplasia increased with time between adenoma removal and the surveillance colonoscopy, from 13% (2.0–3.9 years after polypectomy) to 32% (eight or more years) (Box 2). In our regression analysis, the incidence of advanced neoplasia reached 10% at 2.2 years, 15% at 3.3 years, and 19% at 5.0 years; the projected incidence at ten years was 30%. The estimated incidence was 8.5% one year after non‐advanced adenoma removal, 9.5% after two years, and 13.4% after three years (Box 3). One cancer was diagnosed in a patient with symptoms, 9.8 years after non‐advanced adenoma removal.
In our multivariable analysis, time between the initial and subsequent colonoscopy was positively associated with the incidence of advanced neoplasia (per year: OR, 1.21; 95% CI, 1.08–1.37), as were age at time of non‐advanced adenoma removal (per year: OR, 1.03; 95% CI, 1.01–1.05), two non‐advanced adenomas (v one: OR, 1.74; 95% CI, 1.18–2.57), and prior history of adenoma (OR, 1.48; 95% CI, 1.01–2.15). Sex did not influence the likelihood of advanced neoplasia (online Supporting Information, table 1). The model‐predicted values were not significantly different from the observed values (Pearson goodness‐of‐fit: P = 0.35). No significant interactions between variables were evident.
To assist interpretation of our data in countries in which the definition of advanced neoplasia does not include finding three or four small tubular adenomas or sessile serrated lesions, we performed an analysis in which these cases were not classified as advanced neoplasia. Age, prior adenoma, and time interval between colonoscopies were independently associated with advanced neoplasia at the surveillance colonoscopy, but not the number of adenomas at the time of non‐advanced adenoma diagnosis (Supporting Information, table 2). The estimated incidence of advanced neoplasia with this restricted definition was 10.4% at 5 years and 23.1% at 10 years; the estimated incidence was 4.5% one year after non‐advanced adenoma removal, 5.8% after two years, and 8.3% after three years. The incidence of advanced neoplasia reached 10% at 4.4 years, and 15% at 5.4 years (Supporting Information, figure).
Discussion
We estimated that the incidence of advanced neoplasia following removal of non‐advanced adenoma was 19% at five years and 30% at ten years; applying the more restricted definition of advanced neoplasia used outside Australia, the estimated incidence was 10% at five years and 23% ten years after removal of non‐advanced adenoma. Increasing the colonoscopy surveillance interval from five to ten years would therefore increase the incidence of advanced neoplasia at surveillance by 60% (Australian definition) or 130% (overseas definition).
Setting the appropriate surveillance interval according to an agreed level of risk is difficult. Endoscopy resources are limited in many countries, and evidence‐based determination is needed to optimise their use. Surveillance five years after finding a non‐advanced adenoma at an index procedure is supported by published evidence, with earlier colonoscopy providing no benefit.19,20 Although it has been proposed that extending the surveillance interval for people with non‐advanced adenoma beyond five years is safe,21 few studies have specifically investigated the associated risk. A recent investigation found a low risk of advanced neoplasia in the ten years following removal of non‐advanced adenoma (6.3%),22 but its predominantly male veteran sample may limit the generalisability of its results.
A surveillance time frame of 5–10 years is therefore largely based on expert consensus. The sample in our study more closely reflected real world practice because it included a broader range of patients than many studies. Our finding of increasing risk over time indicates that more studies are needed to confidently determine the degree to which the interval can be extended.
The 2020 European guidelines note that the safety of a longer interval is being investigated.14 The acceptable risk of advanced neoplasia at surveillance colonoscopy is generally regarded as 10–15%.14,15 Our findings suggest that extending the surveillance interval beyond five years markedly increases the risk of advanced neoplasia to levels higher than currently regarded as safe, regardless of the definition of advanced neoplasia applied. Surveillance intervals should reflect clinical practice guidelines based on risk–benefit analyses. Increasing the surveillance interval to ten years after non‐advanced adenoma removal may significantly increase the incidence of advanced neoplasia at follow‐up.
Advanced neoplasia is a heterogeneous pathology group, and the risk of colorectal cancer is unlikely to be the same for all categories. For instance, it is unlikely that three 2 mm tubular adenomas would be associated with the same risk of malignancy as large adenomas with villous features or high grade dysplasia. Two recent evaluations of the risk of advanced neoplasia after non‐advanced adenoma removal found that the risk of advanced neoplasia at follow‐up was greater for patients with small polyps (6–9 mm) than for those with diminutive polyps (1–5 mm), suggesting that further risk stratification may be appropriate.23,24 Although the size of the non‐advanced adenoma was not a significant risk factor in our study, patients who had two tubular adenomas at their initial procedure more frequently had advanced neoplasia on follow‐up than those with one tubular adenoma, providing further evidence that the risk profile for people with non‐advanced adenoma is not uniform.
Including multiple adenomas and sessile serrated lesions in the definition of advanced neoplasia was consistent with Australian guidelines for risk assessment when the colonoscopies in our study were performed. However, to make our findings relevant to overseas guidelines, we also analysed the data after excluding these cases from the definition of advanced neoplasia. Applying this more stringent definition reduced the influence of number of non‐advanced adenomas at baseline colonoscopy on risk of advanced neoplasia, but not that of age, prior history of adenoma, and colonoscopy surveillance interval. The interval is the only modifiable factor in this list, and care should be taken when increasing it beyond five years.
Strengths and limitations
Our analysis included large numbers of procedures with long follow‐up periods, and was one of the first studies to specifically examine advanced neoplasia risk after non‐advanced adenoma over a period of more than five years. Further, we have estimated incidence at different time points on the basis of practice data from a long running surveillance colonoscopy program.
As our study was observational and retrospective in nature, it was difficult to exclude the possibility of selection bias. Further, we had no information about reasons for delayed colonoscopy in some patients. We did not examine metabolic syndrome as a variable, another potential factor for risk stratification.25 In addition, it is possible that incomplete adenoma resection may have increased the risk of advanced neoplasia; as we limited our analysis to good quality colonoscopies, the influence of this factor would have been low. Finally, the incidence of advanced neoplasia could also be influenced by variations in adenoma detection rates, which were not available for the analysed dataset. However, our observations reflect real‐world clinical practice, reflecting the range of skills required for adenoma detection at colonoscopy.
Conclusion
Increasing the surveillance colonoscopy interval after detection of non‐advanced adenoma beyond five years is associated with increased subsequent risk of advanced neoplasia. As stated in the most recent Australian surveillance guidelines,7 one needs to consider the quality of the initial colonoscopy, patient risk factors, and the results of other tests, such as interval faecal immunochemical tests, before lengthening the interval between colonoscopies. It is also important to balance the risks of colonoscopy against the benefits of reducing the risk of advanced neoplasia. Finally, more evidence about the safety of different surveillance intervals in clinical practice is needed to guide clinical care for people at elevated risk of colorectal cancer.
Box 2 – Patient characteristics at baseline colonoscopy (non‐advanced adenoma found and removed), by detection of advanced neoplasia at surveillance colonoscopy
|
Characteristic |
All procedures |
No advanced neoplasia found |
Advanced neoplasia found* |
P |
|||||||||||
|
|
|||||||||||||||
|
Number of procedures |
965 |
796 |
169 |
|
|||||||||||
|
Sex |
|
|
|
0.05 |
|||||||||||
|
Men |
570 [59.1%] |
459 (80.5%) |
111 (20%) |
|
|||||||||||
|
Women |
395 [40.9%] |
337 (85.3%) |
58 (15%) |
|
|||||||||||
|
Age (years), median (IQR) |
62.0 [54.0–69.0] |
61.0 (53.0–68.0) |
64.0 (56.5–72.0) |
< 0.001 |
|||||||||||
|
History of adenoma |
|
|
|
0.010 |
|||||||||||
|
No |
700 [72.5%] |
591 (84.4%) |
109 (16%) |
|
|||||||||||
|
Yes |
265 [27.5%] |
205 (77.4%) |
60 (23%) |
|
|||||||||||
|
Type of previous adenoma |
|
|
|
0.84 |
|||||||||||
|
Advanced adenoma |
156 [58.9%] |
120 (76.9%) |
36 (23%) |
|
|||||||||||
|
Non‐advanced adenoma |
109 [41.1%] |
85 (78%) |
24 (22%) |
|
|||||||||||
|
Colonoscopy number when non‐advanced adenoma diagnosed |
|
|
|
0.67 |
|||||||||||
|
1 |
613 [63.5%] |
511 (83.4%) |
102 (17%) |
|
|||||||||||
|
2 |
197 [20.4%] |
162 (82.2%) |
35 (18%) |
|
|||||||||||
|
3 |
94 [9.7%] |
74 (79%) |
20 (21%) |
|
|||||||||||
|
4 |
35 [3.6%] |
28 (80%) |
7 (20%) |
|
|||||||||||
|
5 |
12 [1.2%] |
10 (83%) |
2 (17%) |
|
|||||||||||
|
6 |
8 [0.8%] |
5 (62%) |
3 (38%) |
|
|||||||||||
|
7 |
4 [0.4%] |
4 (100%) |
0 |
|
|||||||||||
|
8 |
2 [0.2%] |
2 (100%) |
0 |
|
|||||||||||
|
Non‐advanced adenoma: location |
|
|
|
0.10 |
|||||||||||
|
Proximal |
395 [41.7%] |
325 (82.3%) |
70 (18%) |
|
|||||||||||
|
Distal |
362 [38.2%] |
304 (84.0% |
58 (16%) |
|
|||||||||||
|
Rectum |
118 [12.5%] |
100 (84.8%) |
18 (16%) |
|
|||||||||||
|
More than one site |
72 [7.6%] |
52 (72%) |
20 (28%) |
|
|||||||||||
|
Missing data |
18 |
15 |
3 |
|
|||||||||||
|
Non‐advanced adenoma: largest size |
|
|
|
0.68 |
|||||||||||
|
< 5 mm |
508 [53.2%] |
422 (83.1%) |
86 (17%) |
|
|||||||||||
|
5–9 mm |
446 [46.8%] |
366 (82.1%) |
80 (18%) |
|
|||||||||||
|
Missing data |
11 |
8 |
3 |
|
|||||||||||
|
Number of adenomas detected at baseline event |
|
|
|
0.001 |
|||||||||||
|
1 |
775 [80.3%] |
655 (84.5%) |
120 (16%) |
|
|||||||||||
|
2 |
190 [19.7%] |
141 (74.2%) |
49 (26%) |
|
|||||||||||
|
Time until subsequent colonoscopy (years), median (IQR) |
5.2 [4.4‒6.0] |
5.1 (4.4‒6.0) |
5.2 (4.5‒6.2) |
0.05 |
|||||||||||
|
Time until subsequent colonoscopy (years) |
|
|
|
0.047 |
|||||||||||
|
2.0–3.9 |
165 [17.1%] |
144 (87.3%) |
21 (13%) |
|
|||||||||||
|
4.0–5.9 |
553 [57.3%] |
459 (83.0%) |
94 (17%) |
|
|||||||||||
|
6.0–7.9 |
222 [23.0%] |
176 (79.3%) |
46 (21%) |
|
|||||||||||
|
8.0 or more |
25 [2.6%] |
17 (68%) |
8 (32%) |
|
|||||||||||
|
|
|||||||||||||||
|
IQR = interquartile range. * Colorectal cancer, adenomas with high grade dysplasia or villous change, adenomas or sessile serrated lesions with size ≥ 10 mm, sessile serrated lesions with dysplasia, or traditional serrated adenomas, or three or more adenomas or sessile serrated lesions. |
|||||||||||||||
Box 3 – Proportions of procedures with advanced neoplasia at surveillance colonoscopy after non‐advanced adenoma removal, by time to surveillance colonoscopy: regression analysis
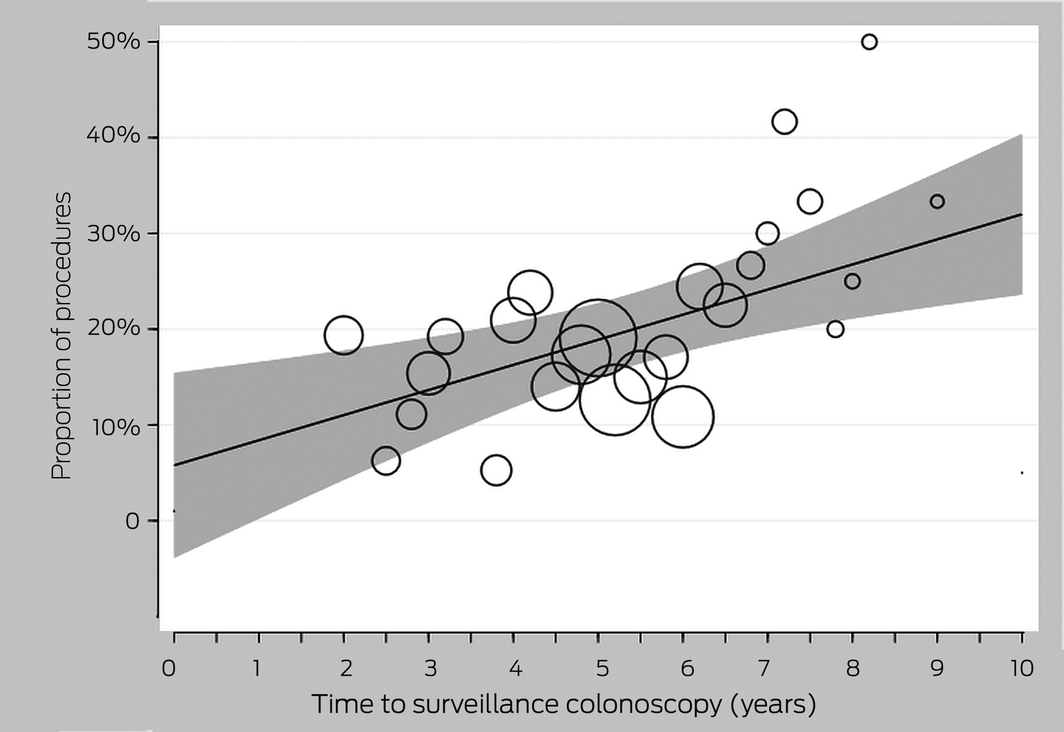
The circles represent mean proportions at each time point; the size of the circle indicates the number of procedures at that time point. The shaded envelope indicates the 95% confidence intervals.
Received 16 July 2020, accepted 15 April 2021
- Zaki Hamarneh1
- Charles Cock1
- Graeme P Young2
- Peter A Bampton3
- Robert Fraser1,4
- Fang LI Ang5
- Feruza Kholmurodova6
- Erin L Symonds1,2
- 1 Flinders Medical Centre, Adelaide, SA
- 2 Flinders Centre for Innovation in Cancer, Flinders University, Adelaide, SA
- 3 Royal Adelaide Hospital, Adelaide, SA
- 4 Flinders University, Adelaide, SA
- 5 College of Medicine and Public Health, Flinders University, Adelaide, SA
- 6 Flinders Centre for Epidemiology and Biostatistics, Flinders University, Adelaide, SA
The study was funded by the Flinders Foundation (Adelaide). Feruza Kholmurodova was supported by a grant provided by the Cancer Council SA Beat Cancer Project.
No relevant disclosures.
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–386.
- 2. Cole SR, Tucker GR, Osborne JM, et al. Shift to earlier stage at diagnosis as a consequence of the National Bowel Cancer Screening Program. Med J Aust 2013; 198: 327–330. https://www.mja.com.au/journal/2013/198/6/shift‐earlier‐stage‐diagnosis‐consequence‐national‐bowel‐cancer‐screening
- 3. Hewitson P, Glasziou P, Irwig L, et al. Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev 2007; CD001216.
- 4. Wiegering A, Ackermann S, Riegel J, et al. Improved survival of patients with colon cancer detected by screening colonoscopy. Int J Colorectal Dis 2016; 31: 1039–1045.
- 5. Citarda F, Tomaselli G, Capocaccia R, et al; Italian Multicentre Study Group. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut 2001; 48: 812–815.
- 6. Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol 2009; 7: 770–775.
- 7. Barclay K, Leggett B, Macrae F, et al; Cancer Council Australia Surveillance Colonoscopy Guidelines Working Party. Updated 25 Mar 2019. Colonoscopic surveillance after polypectomy. https://wiki.cancer.org.au/australia/Guidelines:Colorectal_cancer/Colonoscopy_surveillance/Colonoscopic_surveillance_after_polypectomy (viewed Jan 2021).
- 8. Atkin WS, Morson BC, Cuzick J. Long‐term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992; 326: 658–662.
- 9. Cottet V, Jooste V, Fournel I, et al. Long‐term risk of colorectal cancer after adenoma removal: a population‐based cohort study. Gut 2012; 61: 1180–1186.
- 10. Cancer Council Australia Colonoscopy Surveillance Working Party. Management of epithelial polyps: colonoscopic surveillance after polypectomy. In: Clinical practice guidelines for surveillance colonoscopy in adenoma follow‐up; following curative resection of colorectal cancer; and for cancer surveillance in inflammatory bowel disease. Dec 2011. https://gastros.com.au/wp‐content/uploads/2017/11/cpgcs.pdf (viewed Jan 2021).
- 11. Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi‐Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143: 844–857.
- 12. Hassan C, Quintero E, Dumonceau JM, et al; European Society of Gastrointestinal Endoscopy. Post‐polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2013; 45: 842–851.
- 13. Dubé C, Yakubu M, McCurdy BR, et al. Risk of advanced adenoma, colorectal cancer, and colorectal cancer mortality in people with low‐risk adenomas at baseline colonoscopy: a systematic review and meta‐analysis. Am J Gastroenterol 2017; 112: 1790–1801.
- 14. Rutter MD, East J, Rees CJ, et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post‐polypectomy and post‐colorectal cancer resection surveillance guidelines. Gut 2020; 69: 201–223.
- 15. Good NM, Macrae FA, Young GP, et al. Ideal colonoscopic surveillance intervals to reduce incidence of advanced adenoma and colorectal cancer. J Gastroenterol Hepatol 2015; 30: 1147–1154.
- 16. Bampton PA, Sandford JJ, Young GP. Applying evidence‐based guidelines improves use of colonoscopy resources in patients with a moderate risk of colorectal neoplasia. Med J Aust 2002; 176: 155–157. https://www.mja.com.au/journal/2002/176/4/applying‐evidence‐based‐guidelines‐improves‐use‐colonoscopy‐resources‐patients
- 17. Jenkins M, Lee‐Bates B, Ait Quakrim D, et al; Cancer Council Australia Colorectal Cancer Guidelines Working Party. Colorectal cancer risk according to family history. Updated 30 Oct 2018. https://wiki.cancer.org.au/australia/Clinical_question:Family_history_and_CRC_risk (viewed Jan 2021).
- 18. Snover DC, Ahnen D, Burt R, Odze RD. Serrated polyps of the colon and rectum and serrated polyposis. In: Bosman FT, Carneiro F, Hruban RH, Theise N, editors. WHO classification of tumours of the digestive system. 4th edition. Lyon: International Agency for Research on Cancer (IARC), 2010; pp. 160–165.
- 19. Anderson JC, Baron JA, Ahnen DJ, et al. Factors associated with shorter colonoscopy surveillance intervals for patients with low‐risk colorectal adenomas and effects on outcome. Gastroenterology 2017; 152: 1933–1943.e5.
- 20. Xu M, Wang S, Cao H, et al. Low rate of advanced adenoma formation during a 5‐year colonoscopy surveillance period after adequate polypectomy of non‐advanced adenoma. Colorectal Dis 2016; 18: 179–186.
- 21. Chung SJ, Kim YS, Yang SY, et al. Five‐year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut 2011; 60: 1537–1543.
- 22. Lieberman D, Sullivan BA, Hauser ER, et al. Baseline colonoscopy findings associated with 10‐year outcomes in a screening cohort undergoing colonoscopy surveillance. Gastroenterology 2020; 158: 862–874.e8.
- 23. Hartstein JD, Vemulapalli KC, Rex DK. The predictive value of small versus diminutive adenomas for subsequent advanced neoplasia. Gastrointest Endosc. 2020; 91: 614–621.e6.
- 24. Sneh Arbib O, Zemser V, Leibovici Weissman Y, et al. Risk of advanced lesions at the first follow‐up colonoscopy after polypectomy of diminutive versus small adenomatous polyps of low‐grade dysplasia. Gastrointest Endosc 2017; 86: 713–721.e2.
- 25. Chiu HM, Lee YC, Tu CH, et al. Effects of metabolic syndrome and findings from baseline colonoscopies on occurrence of colorectal neoplasms. Clin Gastroenterol Hepatol 2015; 13: 1134–1142.e8.





Abstract
Objectives: To investigate the incidence of advanced neoplasia (colorectal cancer or advanced adenoma) at surveillance colonoscopy following removal of non‐advanced adenoma; to determine whether the time interval before surveillance colonoscopy influences the likelihood of advanced neoplasia.
Design: Retrospective cohort study.
Setting, participants: Patients enrolled in a South Australian surveillance colonoscopy program with findings of non‐advanced adenoma during 1999–2016 who subsequently underwent surveillance colonoscopy.
Main outcome measures: Incidence of advanced neoplasia at follow‐up surveillance colonoscopy.
Results: Advanced neoplasia was detected in 169 of 965 eligible surveillance colonoscopies (18%) for 904 unique patients (median age, 62.0 years; interquartile range [IQR], 54.0–69.0 years), of whom 570 were men (59.1%). The median interval between the initial and surveillance procedures was 5.2 years (IQR, 4.4–6.0 years; range, 2.0–14 years). Factors associated with increased risk of advanced neoplasia at follow‐up included age (per year: odds ratio [OR], 1.03; 95% CI, 1.01–1.05), prior history of adenoma (OR, 1.48; 95% CI, 1.01–2.15), two non‐advanced adenomas identified at baseline procedure (v one: OR, 1.74; 95% CI, 1.18–2.57), and time to surveillance colonoscopy (OR, 1.21; 95% CI, 1.08–1.37). The estimated incidence of advanced neoplasia was 19% five years after non‐advanced adenoma removal, and 30% at ten years.
Conclusions: Increasing the surveillance colonoscopy interval beyond five years after removal of non‐advanced adenoma increases the risk of detection of advanced neoplasia at follow‐up colonoscopy.