Implementation of any form of population screening for colorectal cancer — faecal occult blood testing, flexible sigmoidoscopy or colonoscopy — will place greater demands on colonoscopy resources.1-6 Reducing the number of colonoscopies currently performed would make resources available for such a screening program.
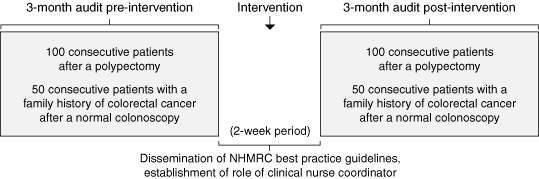
In 1999, after an evidence-based review, the Australian National Health and Medical Research Council (NHMRC) released best-practice guidelines for the prevention, management and detection of colorectal cancer.7 Previous studies have suggested that general practitioners do not uniformly comply with national guidelines,8 and we suspect a similar problem of guideline compliance would be found in specialist practice. Accordingly, we determined whether monitoring the application of NHMRC recommendations for colonoscopic follow-up in two groups of patients with moderate risk of colorectal cancer (those who have had a polyp excised at colonoscopy and those with a family history of colorectal cancer) would reduce the number of surveillance colonoscopies being performed in a public hospital.
The timing of a repeat colonoscopy after a polypectomy; and
The timing of colonoscopy for patients with an "above average" risk of colorectal cancer, based on family history of the disease. (Patients from families with hereditary non-polyposis colorectal cancer [HNPCC] or familial adenomatous polyposis [FAP] were not included.)
For the timing of repeat colonoscopy after a complete polypectomy, NHMRC guidelines recommend a colonoscopy interval of 5 years for patients with one or two small (< 10 mm) tubular adenomas; and 3 years if there were more than two tubular adenomas, or if the adenoma measured 10 mm or more, or if histological examination showed a villous component.
In families with a history of colorectal cancer in a first-degree relative (< 55 years of age), the NHMRC recommends 5-yearly colonoscopy surveillance starting at age 50, or 10 years younger than the index case. For those with a first-degree relative affected over the age of 55, and no other affected relatives, the NHMRC does not recommend colonoscopic screening.
The 100 consecutive patients referred before and after the intervention had similar mean ages and sex distributions (57 men and 43 women aged 63 [SD, 11.3] years v 59 men and 41 women aged 62 [SD, 11.1] years). After the intervention, the proportion of surveillance decisions matching the guidelines increased from 38% to 96% (P < 0.05, χ2 test). The reasons for the change were: repeat colonoscopy was requested too soon after polypectomy (37% of patients); a repeat colonoscopy was requested too late after polypectomy (1% of patients); or the decision was deferred until clinic review, the patient was lost to follow up or no decision was made (24% of patients) (Box 2). The mean time to repeat colonoscopy after polypectomy increased from 2.7 to 3.5 years (P = 0.005; unpaired t-test; 95% CI, 2.69–14.60).
The 50 consecutive patients referred before and after the intervention had similar mean ages and sex distributions (16 men and 34 women, aged 53 [SD, 10.5] years v 19 men and 31 women, aged 50 [SD, 11.2] years). After the intervention, the proportion of surveillance decisions matching the guidelines increased from 62% to 96% (Box 2). In seven patients (14%) referred before the intervention, "family history" was based on only one first-degree relative affected over the age of 55 years. According to the NHMRC guidelines, such patients have average to slightly above average risk and do not need colonoscopic surveillance. There was no significant increase in the mean time to repeat colonoscopy in this group (from 4.7 to 4.8 years). The effect of the intervention in this group was therefore to reduce the number of patients having colonoscopic surveillance rather than increase the time interval for repeat colonoscopy.
To determine the optimal interval between colonoscopies after polypectomy, the NHMRC guidelines rely on a retrospective study of patients from the 1950s and 60s9 and prospective data from the US National Polyp Study.10,11 The recommendation for colonoscopy in patients with a family history of colorectal cancer is accorded Level 3 evidence by the NHMRC guidelines, and is supported by cohort studies.12-15 The guidelines do not recommend colonoscopy screening if the subject has only one affected relative aged 55 years or over, because of a low yield of neoplasia from surveillance in this group.16-18
Received 10 May 2001, accepted 18 January 2002
- Peter A Bampton1
- Jayne J Sandford2
- Graeme P Young3
- Department of Gastroenterology and Hepatology, Flinders Medical Centre, Bedford Park, SA.
The SCOOP Program (Southern Co-operative Program for the Prevention of Colorectal Cancer) was funded through the National Demonstration Hospitals Program Phase 3, a Commonwealth Department of Health and Aged Care initiative, and was supported by the Anti-Cancer Foundation of South Australia.
We would like to thank the following people for their input and support: Dr Daniel Byrne, Dr Pam Rachootin, Dr Richard Johns (Southern Division of General Practice), Ms Robyn Popplewell and Ms Di Rogowski (NHDP 3 Project Office, Flinders Medical Centre), as well as the gastroenterologists and colorectal surgeons at Flinders Medical Centre, all of whom agreed to participate in the study.
- 1. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 1993; 328: 1365-1371.
- 2. Hardcastle JD, Chamberlain JO, Robinson MHE, et al. Randomised controlled trial of faecal occult blood screening for colorectal cancer. Lancet 1996; 348: 1472-1477.
- 3. Kronberg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal occult blood test. Lancet 1996; 348: 1467-1471.
- 4. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999; 91: 434-437.
- 5. St John DJB, Young GP, Alexeyeff MA, et al. Evaluation of new occult blood tests for detection of colorectal neoplasia. Gastroenterology 1993; 104: 1661-1668.
- 6. Atkin WS, Edwards R, Wardle J, et al. UK flexible sigmoidoscopy screening trial: compliance, yield and adverse effects. Gastroenterology 2000; 118: A187.
- 7. National Health and Medical Research Council. Guidelines for the prevention, early detection and management of colorectal cancer. Canberra: NHMRC 1999.
- 8. Olynyk JK, Aquila S, Platell CF, et al. Colorectal cancer screening by general practitioners: comparison with national guidelines. Med J Aust 1998; 168: 331-334.
- 9. Atkin WS, Morson BC, Cuzick J. Long term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med 1992; 326: 658-662.
- 10. Winawer SJ, Zauber AG, O'Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. N Engl J Med 1993; 328: 901-906.
- 11. Zauber AG, Winawer SJ, Bond JH, et al. Can surveillance intervals be lengthened following colonoscopic polypectomy? Gastroenterology 1997; 112: A50.
- 12. Grossman S, Milos ML. Colonoscopic screening of persons with suspected risk factors for colon cancer. I: family history. Gastroenterology 1988; 94: 395-400.
- 13. Hunt LM, Rooney PS, Hardcastle JD et al. Endoscopic screening of relatives of patients with colorectal cancer. Gut 1988; 42: 71-75.
- 14. Pariente A, Milan C, Lafon J, Faivre J. Colonoscopic screening in first degree relatives with "sporadic" colorectal cancer: a case control study. Gastroenterology 1998; 115: 7-15.
- 15. Dowling DJ, St John JB, Macrae F, Hopper JL. Yield from colonoscopic screening in people with a strong family history of common colorectal cancer. J Gastroenterol Hepatol 2000; 15: 939-944. /LI>
- 16. Sondergaard JO, Bulow S, Lynge E. Cancer incidence among parents of patients with colorectal cancer. Int J Cancer 1991; 47: 202-206.
- 17. St John DJB, McDermott FT, Hopper Jl, et al. Cancer risk in relatives of patients with common colorectal cancer. Ann Intern Med 1993; 118: 785-790.
- 18. Fuchs CS, Giovannucci EL, Colditz GA, et al. A prospective study of family history and the risk of colorectal cancer. N Engl J Med 1994; 331: 1669-1674.





Abstract
Objectives: To determine whether applying National Health and Medical Research Council (NHMRC) guidelines for colorectal cancer prevention would reduce the number of follow-up colonoscopies.
Design: A prospective audit of colonoscopic surveillance decisions before and after the intervention.
Setting: The endoscopy suite at a metropolitan tertiary hospital three months before and after January 2000.
Intervention: Dissemination of NHMRC guidelines, and supervision of application of the guidelines by a nurse coordinator.
Subjects: We compared colonoscopic surveillance decisions before and after the intervention in two groups of 100 consecutive patients after polypectomy and in two groups of 50 consecutive patients with a family history of colorectal cancer after a normal colonoscopy.
Main outcome measures: Change in concordance of decisions with NHMRC guidelines; and effect on number of follow-up colonoscopies.
Results: After the intervention, the proportion of postpolypectomy surveillance decisions matching the guidelines increased from 37% to 96% (P < 0.05). The mean time to repeat colonoscopy after polypectomy increased from 2.7 to 3.5 years (P < 0.005) (ie, a 23% reduction in the number of postpolypectomy surveillance colonoscopies performed per year). Likewise, the proportion of family-history surveillance decisions matching the guidelines increased from 63% to 96%. Adhering to the guidelines resulted in a 17% reduction in colonoscopies performed on the basis of a family history of colorectal cancer.
Conclusions: Supervised application of evidence-based guidelines to a colorectal cancer surveillance program significantly reduces the number of surveillance colonoscopies performed.