Paracetamol (acetaminophen) is the most commonly used analgesic medicine;1 because of its low cost and availability without prescription, it is the drug most frequently taken for self‐medication.2 The World Health Organization includes paracetamol in its list of essential medicines (“the most efficacious, safe and cost‐effective medicines for priority conditions”).3 The analgesic action of paracetamol has been attributed to its inhibition of the cyclooxygenase (COX) pathway in the central nervous system, reducing the production of pain‐mediating prostaglandins, but it may also enhance endocannabinoid transmission and modulate descending serotonergic inhibitory pathways.4
Clinical practice guidelines recommend regular, time‐limited use of paracetamol for treating mild to moderate acute pain and chronic non‐malignant pain.5,6 Despite the widespread use of paracetamol, concerns have been expressed that it may be ineffective for treating painful conditions7 and perhaps less safe than previously thought.8 However, claims regarding the frequency and severity of adverse events are largely based on observational studies in which comparatively large therapeutic doses were consumed over long periods (eg, up to 4 g/day for four weeks8).
It has recently been suggested that paracetamol is ineffective or minimally effective for treating low back pain,9 leading to recommendations that non‐steroidal anti‐inflammatory drugs (NSAIDs) be used for initial pharmacological therapy.10 However, there are no published systematic reviews of the efficacy and safety of paracetamol across the broad range of conditions in which it is employed. Further, narrative reviews have included conflicting information, adding to uncertainty about its appropriate use.
Clinicians and patients need information about the efficacy and safety of paracetamol when deciding whether to use it. The aim of our umbrella systematic review was to provide a comprehensive overview of systematic reviews of the efficacy and safety of paracetamol as an analgesic in a range of painful conditions, particularly with respect to providing immediate relief.
Methods
Our search strategy was developed by an experienced researcher‐clinician (author CAS) (Supporting Information, table 1). We searched MEDLINE, EMBASE, PsycINFO, and the Cochrane Database of Systematic Reviews for systematic reviews of randomised controlled trials (RCTs) published in any language in peer‐reviewed journals between 1 January 2010 and 30 April 2020 (the date of our final search). Earlier reviews were not included because they were likely to be incomplete or to have used out‐of‐date approaches for appraising to synthesising trial data. We also included systematic reviews that could not identify any relevant RCTs, and we screened reference lists of published RCTs and systematic reviews for further relevant publications. Our systematic review was prospectively registered with PROSPERO (reference, CRD42015029282; 15 November 2015).
Inclusion criteria
We included systematic reviews that compared the analgesic effects of paracetamol and placebo (saline solution or sterile water) in people of any age with any painful condition, in which change in pain intensity was reported as an outcome in the source material. We placed no restrictions on the dose, formulation (immediate release, modified release, capsule, tablet, oral suspension, intravenous solution), route of administration (intravenous, oral, rectal), regimen (single or multiple dose), or dosing frequency for paracetamol.
Systematic review selection
Two reviewers (CAS, AD) independently screened article titles and abstracts and read the full text of potentially eligible publications; disagreements were resolved by consensus. If several reviews regarding a condition had been published, we selected the review that included the largest number of eligible studies. We documented any notable differences in findings or conclusions between included and excluded reviews.
Data extraction and management
Two reviewers (CAS, GF) independently extracted treatment effect and adverse events data. The primary outcome was the difference between the analgesic effects of paracetamol and placebo. If several instruments were used to measure pain, we extracted primary pain outcomes as defined in the included review.
We report continuous outcomes as between‐group mean differences (MDs) with 95% confidence intervals (CIs), and dichotomous outcomes as risk ratios (RRs) with 95% CIs. We included continuous outcomes in our main analysis if both continuous and dichotomous outcomes were reported, and converted continuous pain outcomes to a standard 0–10 scale.
Treatment effect estimates were extracted for immediate (less than two weeks), short (two weeks to less than six weeks), intermediate (six weeks to less than 12 months), and long term effects (12 months or more). If several effect estimates were reported for immediate relief (eg, one, four, six hours), we used the estimate for the time point closest to the expected time of maximum drug concentration (2–4 hours after administration).11
We also extracted information about paracetamol dose, form, formulation and regimen. Adverse events, if reported, were extracted as secondary outcomes.
Data synthesis
Two reviewers (CAS, GF) independently assessed the conduct of the included systematic reviews with the 16‐item AMSTAR‐2 checklist;12 disagreements were resolved by consensus.
Two reviewers (CAS, GF) assessed confidence in effect estimates (quality of evidence) according to the Grading of Recommendations Assessment, Development and Evaluation criteria (GRADE) criteria.13 We prepared GRADE ratings for reviews that did not report them; we also checked GRADE ratings provided in reviews and report our assessments when they clearly differed from those in the review. Quality level was initially set to “high” and then downgraded for each of four factors: limitations in study design, inconsistency of results, imprecision, and publication bias.13,14 Further details are included in the online Supporting Information, table 2.
We analysed data by medical condition. If a review reported individual trial results rather than a pooled treatment effect, we computed a pooled treatment effect (when possible) and provided a GRADE rating. Meta‐analyses were conducted in Review Manager 5.4 (Cochrane Collaboration), with data pooled in random effects models.
Sensitivity analysis
As GRADE ratings can be applied differently (eg, review authors may apply one or two downgrades for each domain), we conducted sensitivity analyses to determine the impact of less rigorous application of GRADE criteria (maximum of one downgrade for each domain) to the primary outcome.
Results
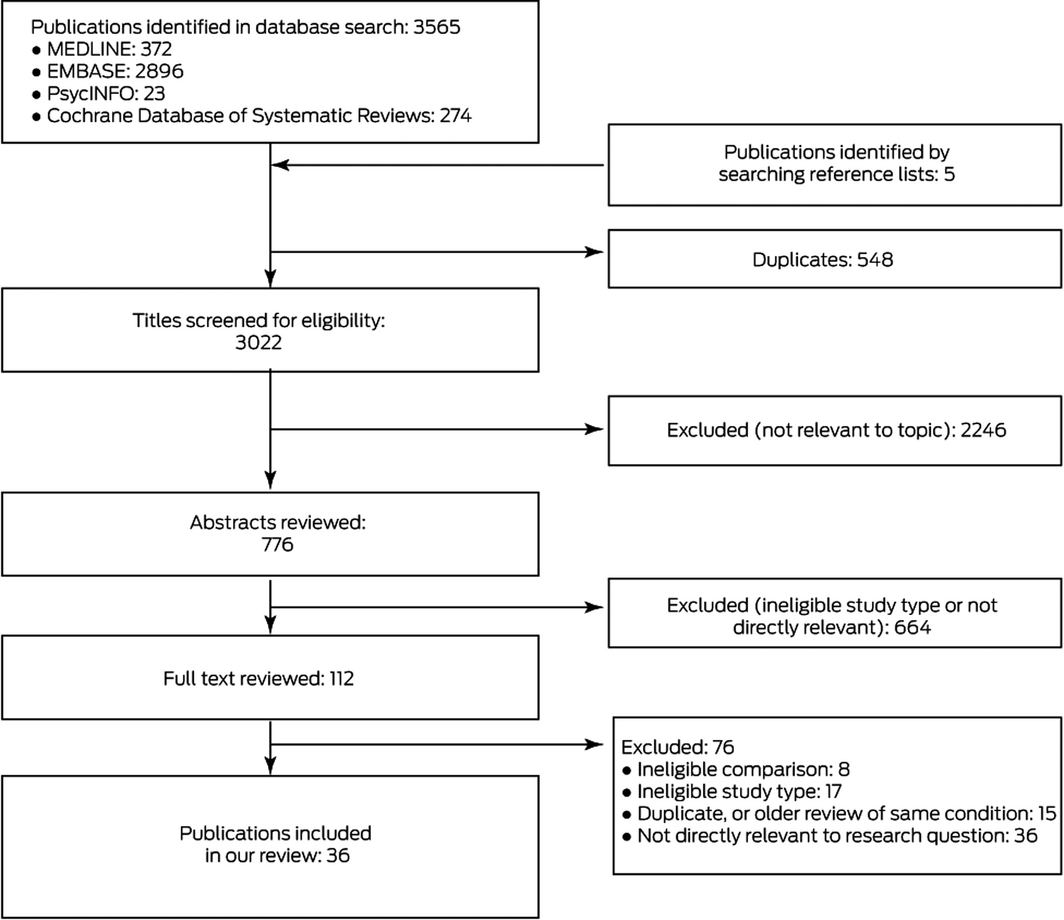
We identified 3570 potentially relevant publications, of which 36 systematic reviews15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50 were ultimately included in our analysis (Box 1; Supporting Information, table 3). Of 112 publications deemed potentially eligible by abstract review, 76 were excluded after reviewing the full text (Box 1; Supporting Information, table 4); the conclusions of excluded reviews that covered the same topic as reviews included in our overview are summarised in Supporting Information, table 5. We excluded a review regarding patients who had undergone knee arthroplasty51 that drew very different conclusions to those of a review selected for our overview42 because it included more eligible trials. Further, we identified an error in a published meta‐analysis that found that intravenous paracetamol was effective for relieving pain 24 hours after bariatric surgery;43 the corrected meta‐analysis52 suggested that paracetamol may not be efficacious in this regard. Overlap between reviews of post‐operative pain and major surgery was limited in the studies included.
The 36 reviews described treatment with paracetamol of 44 painful conditions in adults and children (Box 2). Twenty systematic reviews reported efficacy estimates that we included in our analysis, and we calculated efficacy estimates for 12 reviews; four reviews (paracetamol for treating neuropathic pain,38 hip fracture,41 chronic non‐cancer pain in children,40 and cancer pain in adults39) did not include evidence from RCTs. A comprehensive summary of the converted effect estimates is included in Supporting Information, table 6. Of the 32 reviews including RCT evidence, we provided GRADE ratings for the primary outcome in 26 and revised the GRADE ratings included in four reviews26,29,31,43 (Supporting Information, table 7).
Effect estimates we calculated from original RCT publications or from data in the included reviews are summarised in Supporting Information, table 8. Cochrane risk of bias ratings for individual RCTs was determined when risk of bias was not adequately assessed in the original included reviews; these results are summarised in Supporting Information, table 9. AMSTAR‐2 ratings of the included reviews are summarised in Supporting Information, table 10.
Paracetamol treatment regimens
Twenty‐one of the 36 included systematic reviews evaluated RCTs in which the effects of a single oral or intravenous dose of paracetamol (typically 0.5–1 g) or of the paracetamol pro‐drug propacetamol were assessed. As most systematic reviews assessed immediate term pain responses (a few hours to two weeks after administration), we discuss immediate term effects only. The two exceptions are osteoarthritis pain44 and rheumatoid arthritis,16 for which paracetamol was administered as part of a continuing course of treatment lasting a few days to several weeks or months. Sustained release tablets for acute low back pain were specifically evaluated,28 but reported information on paracetamol formulation was otherwise limited.
High quality evidence of efficacy
For two conditions, there was high quality evidence that paracetamol (1 g, up to four times per day) is more efficacious than placebo, but the effect sizes were very small (less than one point on a 0–10 scale). A systematic review of patients with knee or hip osteoarthritis (five RCTs, 1686 patients) found that paracetamol (4 × 1 g/day for up to 12 weeks) provided mean pain relief of 0.3 points on a 0–10‐point pain scale (95% CI, −0.6 to −0.1 points).44 Another review (four RCTs, 453 patients) found that paracetamol (4 × 1 g/day for 24 hours) reduced pain after craniotomy (MD, –0.8 points; 95% CI, –1.4 to –0.3 points)49 (Box 3).
Moderate quality evidence of efficacy
For two conditions, there is moderate quality evidence that paracetamol is more efficacious than placebo. One systematic review (six RCTs, 797 patients) found that paracetamol (1 g, single dose) provided pain relief for women with early post partum perineal pain (patients achieving 50% pain relief: RR, 2.4; 95% CI, 1.5–3.8); lower doses (500–650 mg, single dose) were also effective (RR, 1.9; 95% CI, 1.2–2.9).17 Another review (eight RCTs, 5890 participants) found that paracetamol (up to 1 g, single or multiple doses) was superior to placebo for relieving pain in people with episodic tension‐type headache (pain‐free at 2 hours: RR, 1.3; 95% CI, 1.1–1.4)31 (Box 4).
Low quality evidence of efficacy
Low quality evidence is available that paracetamol is efficacious in eleven painful conditions: dental procedures (bonding and separation),36 major surgery (including abdominal, neurosurgical, gynaecological, and orthopaedic surgery; single dose regimen),35 acute migraine in adults,18 otitis media in children,30 orbital surgery,46 renal colic,34 metastatic breast cancer,46 common cold‐related headache,20 pain 30 minutes after hysterosalpingography,24 cataract surgery,19 and abdominal pain unrelated to surgery29 (Box 3, Box 4).
High quality evidence of no efficacy
One systematic review28 found high quality evidence that oral paracetamol (up to 3.99 g per day for up to four weeks) was not superior to placebo for treating acute low back pain (one RCT, 1643 patients; MD, 0.2 points; 95% CI –0.1 to 0.4 points) (Box 3).
Low quality evidence of no efficacy
Evidence that paracetamol is not superior to placebo for relieving the pain of sore throat in people with common colds,20 migraine in children and adolescents,27 pain during hysterosalpingography,24 pain in newborns,50 dental surgery in children,25 uterine cramping/involution after birth,15 or reconstructive vaginal surgery46 is of low quality (Box 3, Box 4).
Very low quality evidence (inconclusive or no evidence)
Very low quality evidence was deemed inconclusive, even if the effect estimate was statistically significant. Evidence regarding the value of paracetamol was insufficient to guide treatment in seventeen pain conditions for which RCT evidence was available: chronic low back pain,28 post‐caesarean delivery pain45 (multiple‐dose regimens), prevention of post‐operative pain at rest,23 endodontic surgery pain,37 knee and hip arthroplasty42 (single or multiple dose regimens), abdominal surgery32 (multiple dose regimens), rheumatoid arthritis,16 hip fracture,41 tonsillectomy in adults,47 laparoscopic cholecystectomy,21 catheter‐related bladder discomfort,22 myringotomy in children,48 bariatric surgery,43 cardiac surgery,33 and diverse post‐operative pain conditions (thyroidectomy, lower extremity, lumbar disk, nephrolithotomy)26 (Box 3). Evidence regarding its value in four conditions without RCT evidence was also very low quality: non‐cancer pain in children and adolescents,40 neuropathic pain,38 cancer pain in adults,39 and hip fracture.41
Adverse events
Adverse events data were assessed in 21 systematic reviews (Supporting Information, table 11).15,16,17,18,19,33,34,35,43,44,45,46,48 One systematic review found moderate level evidence that the risk of transiently elevated liver enzymes (two weeks to three months after administration) was greater for paracetamol than placebo in patients with spinal pain or osteoarthritis (RR, 3.8; 95% CI, 1.9–7.4);44 the clinical implications of this finding, however, were unclear. The other systematic reviews found that frequency of any or serious adverse events were similar for paracetamol and placebo, but the evidence was generally of low quality.
Sensitivity analysis
GRADE ratings derived with a less rigorous approach are summarised in Supporting Information, table 12; they do not differ markedly from those of our main analysis.
Discussion
High or moderate quality evidence that paracetamol (typically 0.5–1 g, single or multiple doses) is superior to placebo for relieving pain was available for only four of 44 painful conditions examined: knee and hip osteoarthritis, craniotomy, tension headache, and perineal pain. The effect sizes were modest, particularly for patients with knee or hip osteoarthritis or tension headache. The frequency of adverse events (any or serious) was similar for paracetamol and placebo, although transiently elevated blood levels of liver enzymes (three times the normal limit) were documented in patients with spinal pain or osteoarthritis treated with paracetamol.44 However, most studies evaluated the immediate effects of single doses of paracetamol, which does not reflect typical clinical use.
Our review of systematic reviews provides greater clarity about the efficacy of paracetamol in conditions for which conflicting evidence has been reported. For some conditions, we identified several relevant systematic reviews. One review on knee and hip arthroplasty51 reported different findings to those of the review we included;42 we determined the reasons for this discordance, and resolved it by analysing the data from eligible placebo‐controlled trials. We found that evidence for the effectiveness of multiple or single dose paracetamol therapy after knee and hip arthroplasty is inconclusive.
Implications for clinicians, patients and policy makers
Evidence for the efficacy of paracetamol in most pain conditions is of low quality or inconclusive, and for the four conditions for which there is high or moderate quality evidence of efficacy, the benefits are small. However, many trials evaluated single doses or short courses of paracetamol, unlike typical clinical practice, while others did not choose assessment time points that corresponded to the maximum blood concentration of paracetamol.11 Reported efficacy estimates may consequently be low.
The frequency of adverse events was similar for patients receiving paracetamol and placebo. However, this conclusion is largely based on single‐dose studies, and cannot inform judgements about the risk of harm associated with long term use of paracetamol by patients with chronic pain conditions. Further, reporting of adverse events, particularly long term events, is often incomplete in randomised controlled trials because of short follow‐up periods. Evidence regarding the safe duration of paracetamol use is inconclusive and based on low quality evidence from observational studies with significant risk of confounding.53 Nevertheless, clinicians should monitor their patients for possible signs of paracetamol‐related adverse events, including increased blood levels of liver enzymes, particularly when treating chronic pain with paracetamol. Further, the risk of harm should be considered when recommending paracetamol to older people, especially those who are frail or have impaired liver function.
We found low quality evidence for the benefits of paracetamol in conditions typically associated with severe pain, including renal colic and abdominal pain. One review found that the benefit of 1 g intravenous paracetamol for people with renal colic was similar to that of opioid analgesics or NSAIDs.54 This was surprising, as it is generally believed that these analgesics are more potent than paracetamol, and that the specific effect of NSAIDs on prostaglandin synthesis relieves the smooth muscle spasms characteristic of renal colic.55 Rescue opioid medication for post‐operative pain has been reported to be less frequently required by patients taking paracetamol than by those using NSAIDs.56
“Clinically important difference” is often defined in pain research as being a 10% change in pain level or a one‐point change on a 0–10‐point pain scale, but these are arbitrary thresholds. Physicians should discuss the clinical importance of effect estimates with their patients, as it will depend upon their baseline health status, individual circumstances, cost, risk of harm, and convenience of treatment.
Strengths and limitations of our review
We used a comprehensive search strategy, report quantitative estimates of treatment effects (including estimates for systematic reviews that did not report them), and determined the overall quality of evidence according to the GRADE criteria. Further, we included a corrected meta‐analysis,52 as we noted errors in the original review.
GRADE ratings can be applied using different approaches and there is currently no consensus about which is preferable. Nevertheless, we allowed up to two downgrades for each domain (except publication bias), as recommended in the GRADE handbook.13 Less rigorous approaches (eg, applying a single downgrade for each domain when appropriate) did not markedly alter our conclusions that the benefits of paracetamol for most painful conditions have not been established. However, GRADE ratings for heterogeneity and publication bias could not be assessed for many outcomes. Further, for half the conditions evaluated, the systematic reviews we included identified only single eligible studies, which limits interpretation of their findings.
Our overview is the most comprehensive, up‐to‐date, and reliable synthesis of information on paracetamol efficacy for treating painful conditions. Previous overviews were more limited in scope, often restricted to single conditions or to Cochrane reviews.57 Further, the most recent overview, published in 2015, was based on the findings of six systematic reviews published during 2002–2010.58 In contrast, we included 36 Cochrane and non‐Cochrane reviews, of which 26 were published after 2016.
Conclusion
Our review highlights the need for large, high quality trials to reduce uncertainty about the efficacy of paracetamol for relieving common pain conditions. Available evidence is largely derived from trials that evaluated the effects of single doses; investigations of multiple dose regimens, reflecting usual practice, are needed. For some long term conditions, such as osteoarthritis, long term efficacy and safety should also be evaluated.
While paracetamol is widely used, its efficacy in relieving pain has been established for only a handful of conditions, and its benefits are often modest. Although some trials have evaluated regimens that may have underestimated its utility, the clinical application of paracetamol is primarily guided by low quality evidence, at best.
Box 2 – Systematic reviews of randomised, placebo‐controlled trials investigating the efficacy of paracetamol for treating pain, by finding, medical condition, and quality of evidence (GRADE)
|
Reviewed condition, by finding (v placebo) |
Paracetamol dose |
Trials |
Total participants |
Evidence quality* |
|||||||||||
|
|
|||||||||||||||
|
Efficacious |
|
|
|
|
|||||||||||
|
Single dose regimens |
|
|
|
|
|||||||||||
|
Perineal pain17 |
Oral (probable); 0.5–1 g |
6 |
797 |
Moderate |
|||||||||||
|
Abdominal pain (unrelated to surgery)29 |
Intravenous; 15 mg/kg over 3 min |
1 |
210 |
Low |
|||||||||||
|
Acute migraine in adults18 |
Oral; 1 g |
3 |
717 |
Low |
|||||||||||
|
Renal colic34 |
Intravenous; 1 g |
1 |
152 |
Low |
|||||||||||
|
Orbital surgery46 |
Intravenous; 1 g |
1 |
150 |
Low |
|||||||||||
|
Common cold‐related achiness20 |
Oral; 0.5–1 g |
1 |
379 |
Low |
|||||||||||
|
Common cold‐related headache20 |
Oral; 0.5–1 g |
1 |
379 |
Low |
|||||||||||
|
Pain after hysterosalpingography24 |
1 g, 30 min before procedure (form unreported) |
1 |
88 |
Low |
|||||||||||
|
Dental procedures (eg, bonding and separation)36 |
Oral; 0.5–0.65 g, 1 h before procedure and up to four doses after procedure |
4 |
107 |
Low |
|||||||||||
|
Post‐operative pain (major surgery; including abdominal, neurosurgical, gynaecological, and orthopaedic surgery)35 |
Unspecified |
15 |
> 524 |
Low |
|||||||||||
|
Post‐operative pain (cataract surgery)19 |
Oral; 1 g, single dose 1 h before surgery |
1 |
160 |
Low |
|||||||||||
|
Multiple dose regimens |
|
|
|
|
|||||||||||
|
Knee and hip osteoarthritis44 |
Oral; 4 x 1 g/day; up to 12 weeks |
5 |
1686 |
High |
|||||||||||
|
Craniotomy49 |
Intravenous; 4 x 1 g/day; up to 24 h |
4 |
453 |
High |
|||||||||||
|
Metastatic breast cancer46 |
Intravenous; 4 x 1 g, every 6 h |
1 |
87 |
Low |
|||||||||||
|
Otitis media pain in children30 |
Oral; 3 x 10 mg/kg/day for up to 48 h |
1 |
148 |
Low |
|||||||||||
|
Mixed dose regimens |
|
|
|
|
|||||||||||
|
Episodic tension type headache31 |
Oral; up to 1 g, single or multiple doses |
8 |
5890 |
Moderate |
|||||||||||
|
Not efficacious |
|
|
|
|
|||||||||||
|
Single dose regimens |
|
|
|
|
|||||||||||
|
Sore throat in common cold20 |
Oral; 0.5–1 g |
1 |
379 |
Low |
|||||||||||
|
Migraine in children and adolescents27 |
Oral; 10 mg/kg |
1 |
88 |
Low |
|||||||||||
|
Pain during hysterosalpingography24 |
1 g, 30 min before procedure (form not reported) |
1 |
88 |
Low |
|||||||||||
|
Pain in newborns50 |
Suppositories; 40 mg/kg, 90 min before heel lance |
1 |
38 |
Low |
|||||||||||
|
Post‐operative pain (dental surgery in children)25 |
Oral; 80 mg |
2 |
100 |
Low |
|||||||||||
|
Uterine cramping/involution after birth15 |
Oral; 0.65 g |
1 |
48 |
Low |
|||||||||||
|
Multiple dose regimens |
|
|
|
|
|||||||||||
|
Acute low back pain28 |
Oral; up to 3.99 g daily, for up to 4 weeks |
1 |
1643 |
High |
|||||||||||
|
Reconstructive vaginal surgery46 |
Intravenous; 4 x 1 g every 6 h |
1 |
90 |
Low |
|||||||||||
|
|
|
|
|
|
|||||||||||
|
Inconclusive (very low quality evidence) |
|
|
|
|
|||||||||||
|
Single dose regimens |
|
|
|
|
|||||||||||
|
Catheter‐related bladder discomfort22 |
Intravenous; 15 mg/kg |
1 |
64 |
— |
|||||||||||
|
Early post‐operative pain (0‐4 h post‐operative)23 |
Intravenous; 2 g |
9 |
609 |
— |
|||||||||||
|
Myringotomy in children48 |
Oral; 15 mg/kg |
1 |
43 |
— |
|||||||||||
|
Post‐operative pain (endodontic surgery)37 |
Oral; 0.325–1 g |
2 |
57 |
— |
|||||||||||
|
Post‐operative pain (knee and hip arthroplasty)42 |
Intravenous; 1 g |
1 |
116 |
— |
|||||||||||
|
Preventing late post‐operative pain (24 h post‐operative)23 |
Intravenous; up to 2 g before or at end of surgery |
5 |
328 |
— |
|||||||||||
|
Post‐operative pain (including thyroidectomy, lower extremity, lumbar disk, orthopaedic, nephrolithotomy)26 |
Intravenous; 1 g |
12 |
837 |
— |
|||||||||||
|
Post‐operative pain (knee and hip arthroplasty)42 |
Intravenous; 1 g single dose |
1 |
116 |
— |
|||||||||||
|
Multiple dose regimens |
|
|
|
|
|||||||||||
|
Bariatric surgery43 |
Intravenous; 4 x 1 g every 6 h |
4 |
349 |
— |
|||||||||||
|
Chronic low back pain28 |
Oral; up to 4 g/day |
1 |
72 |
— |
|||||||||||
|
Post‐operative pain (abdominal surgery)32 |
Intravenous; up to 4 g/day |
8 |
793 |
— |
|||||||||||
|
Post‐operative pain (cardiac surgery)33 |
Intravenous; up to 4 g/24 h |
3 |
261 |
— |
|||||||||||
|
Post‐operative pain (knee and hip arthroplasty)42 |
Intravenous; 1 g paracetamol or 2 g propacetamol every 6 h |
2 |
152 |
— |
|||||||||||
|
Mixed dose regimens |
|
|
|
|
|||||||||||
|
Post‐caesarean delivery pain45 |
Intravenous; 1 g, single or multiple doses |
5 |
388 |
— |
|||||||||||
|
Tonsillectomy in adults47 |
Intravenous; 1 g, single dose or every 6 h |
2 |
153 |
— |
|||||||||||
|
Post‐laparoscopic cholecystectomy21 |
Multiple doses up to 3 g over 48 h |
3 |
146 |
— |
|||||||||||
|
Rheumatoid arthritis16 |
Oral; multiple 1 g doses per day, up to 17 days |
2 |
55 |
— |
|||||||||||
|
No evidence from randomised control trials |
|
|
|
|
|||||||||||
|
Cancer pain in adults39 |
— |
|
|
— |
|||||||||||
|
Neuropathic pain38 |
— |
|
|
— |
|||||||||||
|
Non‐cancer pain in children and adolescents40 |
— |
|
|
— |
|||||||||||
|
Early management of hip fracture41 |
— |
|
|
— |
|||||||||||
|
|
|||||||||||||||
|
* According to the Grading of Recommendations Assessment, Development and Evaluation criteria (GRADE) criteria.13 |
|||||||||||||||
Box 3 – Effect estimates for systematic reviews of studies reporting continuous outcomes (mean difference in pain level change, paracetamol v placebo, on 0–10‐point pain scale)
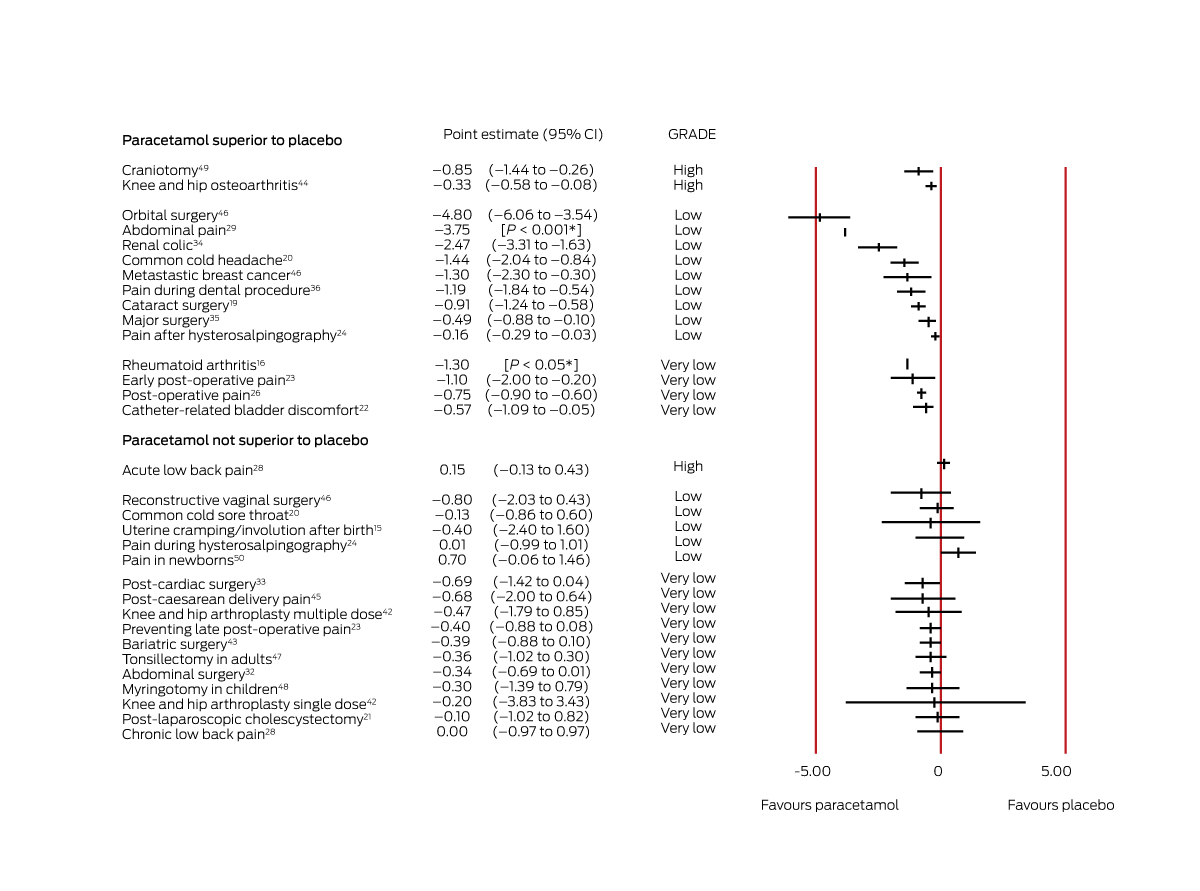
CI = confidence interval; GRADE = evidence quality according to the Grading of Recommendations Assessment, Development and Evaluation criteria (GRADE) criteria.13 *These reviews reported P values for differences, but not 95% CIs.
Box 4 – Effect estimates for systematic reviews of studies reporting dichotomous outcomes (risk ratios). A. Pain relief (risk ratio > 1 favours paracetamol); B. Pain (risk ratio < 1 favours paracetamol)
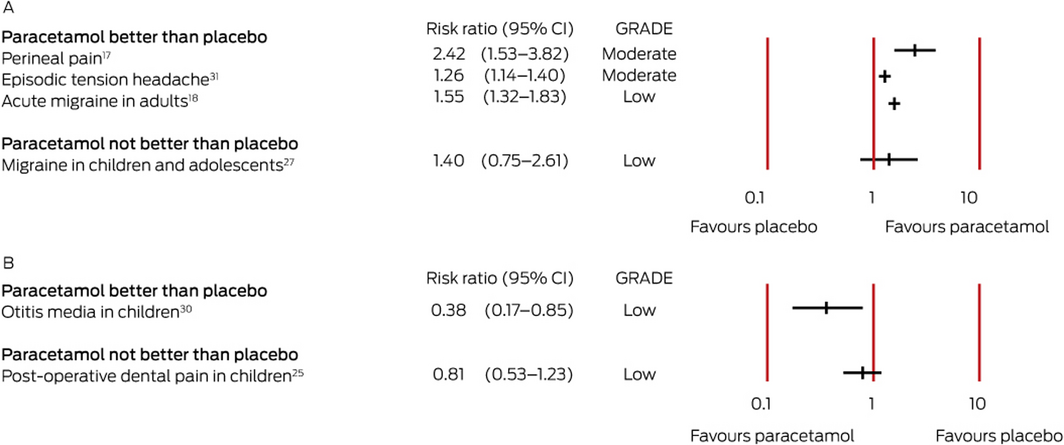
CI = confidence interval; GRADE = evidence quality according to the Grading of Recommendations Assessment, Development and Evaluation criteria (GRADE) criteria.13
Received 21 July 2020, accepted 13 October 2020
- Christina Abdel Shaheed1
- Giovanni E Ferreira2
- Alissa Dmitritchenko3
- Andrew J McLachlan4
- Richard O Day5,6
- Bruno Saragiotto7
- Christine Lin2
- Vicki Langendyk8
- Fiona Stanaway1
- Jane Latimer2
- Steven Kamper2
- Hanan McLachlan2
- Harbeer Ahedi2
- Christopher G Maher1
- 1 The University of Sydney, Sydney, NSW
- 2 Institute for Musculoskeletal Health, University of Sydney, Sydney
- 3 Sutherland Hospital, Sydney, NSW
- 4 Centre for Education and Research on Ageing, University of Sydney, Sydney, NSW
- 5 St Vincent's Hospital, Sydney, NSW
- 6 St Vincent's Clinical School, University of New South Wales, Sydney, NSW
- 7 Universidade Cidade de São Paulo, São Paulo, Brazil
- 8 University of New South Wales, Sydney, NSW
Christina Abdel Shaheed is supported by a University of Sydney Early Career Development Fellowship. Steven Kamper and Christine Lin are supported by National Health and Medical Research Council (NHMRC) fellowships. Bruno Saragiotto is supported by the São Paulo Research Foundation, Brazil. Chris Maher is supported by an NHMRC Principal Research Fellowship (APP1103022), and holds an NHMRC program grant (APP1113532) and two Centre for Research Excellence grants (APP1134856, APP1171459). He has received research grants from several government and not‐for‐profit agencies; his expenses have been covered by professional associations that have invited him to speak. FlexEze provided heat wraps at no cost for the Sydney Health Partners Emergency Department (SHaPED) low back pain treatment trial, in which Chris Maher and Christina Abdel Shaheed are investigators.
Christopher Maher, Richard Day, Andrew McLachlan, Christine Lin, and Jane Latimer were investigators in the PACE study (paracetamol for acute lower back pain; ACTN 12609000966291), funded by the NHMRC and GlaxoSmithKline Australia. The Sydney Pharmacy School receives research funding from GlaxoSmithKline Australia for a research student supervised by Andrew McLachlan.
- 1. Australian Department of Health. Australian statistics on medicine 2015. Updated 18 Nov 2016. https://www.pbs.gov.au/info/statistics/asm/asm-2015 (viewed Mar 2021).
- 2. Wastesson JW, Martikainen JE, Zoëga H, et al. Trends in use of paracetamol in the Nordic countries. Basic Clin Pharmacol Toxicol 2018; 123: 301–307.
- 3. World Health Organization. WHO model list of essential medicines, 21st list. July 2019. https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06 (viewed July 2019).
- 4. Sharma CV, Mehta V. Paracetamol: mechanisms and updates. Continuing Education in Anaesthesia Critical Care and Pain 2014; 14: 153–158.
- 5. Blondell RD, Azadfard M, Wisniewski AM. Pharmacologic therapy for acute pain. Am Fam Physician 2013; 87: 766–772.
- 6. Australian Department of Health, Therapeutic Goods Administration. Paracetamol: practitioner fact sheet. June 2003. https://www.tga.gov.au/alert/paracetamol-practitioner-fact-sheet (viewed Mar 2021).
- 7. Moore A. Paracetamol: widely used and largely ineffective [news item]. Cochrane UK; undated. https://uk.cochrane.org/news/paracetamol-widely-used-and-largely-ineffective (viewed Oct 2018).
- 8. Roberts E, Delgado Nunes V, Buckner S, et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis 2016; 75: 552–559.
- 9. Williams CM, Maher CG, Latimer J, et al. Efficacy of paracetamol for acute low‐back pain: a double‐blind, randomised controlled trial. Lancet 2014; 384: 1586–1596.
- 10. Qaseem A, Wilt TJ, McLean RM, et al; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Inter Med 2017; 166: 514–530.
- 11. Clinical Pharmacology Resources. Onset peak and duration of common pain medications. https://hhs.texas.gov/sites/default/files/documents/doing-business-with-hhs/provider-portal/QMP/PainMedicationTable.pdf (viewed Oct 2018).
- 12. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ 2017; 358: j4008.
- 13. Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook. Updated 2013. https://gdt.gradepro.org/app/handbook/handbook.html (viewed May 2019).
- 14. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634.
- 15. Deussen AR, Ashwood P, Martis R. Analgesia for relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst Rev 2011; CD004908.
- 16. Hazlewood G, van der Heijde DM, Bombardier C. Paracetamol for the management of pain in inflammatory arthritis: a systematic literature review. J Rheumatol Suppl 2012; 90: 11–16.
- 17. Chou D, Abalos E, Gyte GM, Gülmezoglu AM. Paracetamol/acetaminophen (single administration) for perineal pain in the early postpartum period. Cochrane Database Syst Rev 2013; CD008407.
- 18. Derry S, Moore RA. Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database Syst Rev 2013; CD008040.
- 19. Porela‐Tiihonen S, Kaarniranta K, Kokki M, et al. A prospective study on postoperative pain after cataract surgery. Clin Ophthalmol 2013; 7: 1429–1435.
- 20. Li S, Yue J, Dong BR, et al. Acetaminophen (paracetamol) for the common cold in adults. Cochrane Database Syst Rev 2013; CD008800.
- 21. Gurusamy KS, Davidson C, Gluud C, Davidson BR. Early versus delayed laparoscopic cholecystectomy for people with acute cholecystitis. Cochrane Database Syst Rev 2013; CD005440.
- 22. Bai Y, Wang X, Li X, et al. Management of catheter‐related bladder discomfort in patients who underwent elective surgery. J Endourol 2015; 29: 640–649.
- 23. De Oliveira GS, Castro‐Alves LJ, McCarthy RJ. Single‐dose systemic acetaminophen to prevent postoperative pain: a meta‐analysis of randomized controlled trials. Clin J Pain 2015; 31: 86–93.
- 24. Hindocha A, Beere L, O’Flynn H, et al. Pain relief in hysterosalpingography. Cochrane Database Syst Rev 2015; CD006106.
- 25. Ashley PF, Parekh S, Moles DR, et al. Preoperative analgesics for additional pain relief in children and adolescents having dental treatment. Cochrane Database Syst Rev 2016; CD008392.
- 26. McNicol ED, Ferguson MC, Haroutounian S, et al. Single dose intravenous paracetamol or intravenous propacetamol for postoperative pain. Cochrane Database Syst Rev 2016; CD007126.
- 27. Richer L, Billinghurst L, Linsdell MA, et al. Drugs for the acute treatment of migraine in children and adolescents. Cochrane Database Syst Rev 2016; CD005220.
- 28. Saragiotto BT, Machado GC, Ferreira ML, et al. Paracetamol for low back pain. Cochrane Database Syst Rev 2016; CD012230.
- 29. Sin B, Wai M, Tatunchak T, Motov SM. The use of intravenous acetaminophen for acute pain in the emergency department. Acad Emerg Med 2016; 23: 543–553.
- 30. Sjoukes A, Venekamp RP, van de Pol AC, et al. Paracetamol (acetaminophen) or non‐steroidal anti‐inflammatory drugs, alone or combined, for pain relief in acute otitis media in children. Cochrane Database Syst Rev 2016; CD011534.
- 31. Stephens G, Derry S, Moore RA. Paracetamol (acetaminophen) for acute treatment of episodic tension‐type headache in adults. Cochrane Database Syst Rev 2016; CD011889.
- 32. Blank JJ, Berger NG, Dux JP, et al. The impact of intravenous acetaminophen on pain after abdominal surgery: a meta‐analysis. J Surg Res 2018; 227: 234–245.
- 33. Douzjian DJ, Kulik A. Old drug, new route: a systematic review of intravenous acetaminophen after adult cardiac surgery. J Cardiothorac Vasc Anesth 2017; 31: 694–701.
- 34. García‐Perdomo HA, Echeverría‐García F, López H, et al. Pharmacologic interventions to treat renal colic pain in acute stone episodes: systematic review and meta‐analysis. Prog Urol 2017; 27: 654–665.
- 35. Martinez V, Beloeil H, Marret E, et al. Non‐opioid analgesics in adults after major surgery: systematic review with network meta‐analysis of randomized trials. Br J Anaesth 2017; 118: 22–31.
- 36. Monk AB, Harrison JE, Worthington HV, Teague A. Pharmacological interventions for pain relief during orthodontic treatment. Cochrane Database Syst Rev 2017; CD003976.
- 37. Shirvani A, Shamszadeh S, Eghbal MJ, et al. Effect of preoperative oral analgesics on pulpal anesthesia in patients with irreversible pulpitis: a systematic review and meta‐analysis. Clin Oral Investig 2017; 21: 43–52.
- 38. Wiffen PJ, Knaggs R, Derry S, et al. Paracetamol (acetaminophen) with or without codeine or dihydrocodeine for neuropathic pain in adults. Cochrane Database Syst Rev 2016; CD012227.
- 39. Wiffen PJ, Derry S, Moore RA, et al. Oral paracetamol (acetaminophen) for cancer pain. Cochrane Database Syst Rev 2017; CD012637.
- 40. Cooper TE, Fisher E, Anderson B, et al. Paracetamol (acetaminophen) for chronic non‐cancer pain in children and adolescents. Cochrane Database Syst Rev 2017; CD012539.
- 41. Dixon J, Ashton F, Baker P, et al. Assessment and early management of pain in hip fractures: the impact of paracetamol. Geriatr Orthop Surg Rehabil 2018; 9: 2151459318806443.
- 42. Guo H, Wang C, He Y. A meta‐analysis evaluates the efficacy of intravenous acetaminophen for pain management in knee or hip arthroplasty. J Orthop Sci 2018; 23: 793–800.
- 43. Lee Y, Yu J, Doumouras AG, et al. Intravenous acetaminophen versus placebo in post‐bariatric surgery multimodal pain management: a meta‐analysis of randomized controlled trials. Obes Surg 2019; 29: 1420–1428.
- 44. Leopoldino AO, Machado GC, Ferreira PH, et al. Paracetamol versus placebo for knee and hip osteoarthritis. Cochrane Database Syst Rev 2019; CD013273.
- 45. Ng QX, Loke W, Yeo WS, et al. A meta‐analysis of the utility of preoperative intravenous paracetamol for post‐caesarean analgesia. Medicina 2019; 55: 424.
- 46. O’Neill RC, Hayes KD, Davison SP. Safety of postoperative opioid alternatives in plastic surgery: a systematic review. Plast Reconstr Surg 2019; 144: 991–999.
- 47. Tolska HK, Hamunen K, Takala A, Kontinen VK. Systematic review of analgesics and dexamethasone for post‐tonsillectomy pain in adults. Br J Anaesth 2019; 123: e397–e411.
- 48. Campbell HT, Yuhan BT, Smith B, et al. Perioperative analgesia for patients undergoing otologic surgery: an evidence‐based review. Laryngoscope 2020; 130: 190–199.
- 49. Ghaffarpasand F, Dadgostar E, Ilami G, et al. Intravenous acetaminophen (paracetamol) for postcraniotomy pain: systematic review and meta‐analysis of randomized controlled trials. World Neurosurg 2020; 134: 569–576.
- 50. Ohlsson A, Shah PS. Paracetamol (acetaminophen) for prevention or treatment of pain in newborns. Cochrane Database Syst Rev 2020; CD011219.
- 51. Liang L, Cai Y, Li A, Ma C. The efficiency of intravenous acetaminophen for pain control following total knee and hip arthroplasty: a systematic review and meta‐analysis. Medicine (Baltimore) 2017; 96: e8586.
- 52. Abdel Shaheed C, Ferreira GE, Maher CG. Correction to meta‐analysis of intravenous acetaminophen (paracetamol) versus placebo post‐bariatric surgery. Obes Surg 2020; 30: 3583–3584.
- 53. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr Cartilage 2010; 18: 476–499.
- 54. Pathan SA, Mitra B, Cameron PA. A systematic review and meta‐analysis comparing the efficacy of nonsteroidal anti‐inflammatory drugs, opioids, and paracetamol in the treatment of acute renal colic. Eur Urol 2018; 73: 583–595.
- 55. Holdgate A, Pollock T. Systematic review of the relative efficacy of non‐steroidal anti‐inflammatory drugs and opioids in the treatment of acute renal colic. BMJ 2004; 328: 1401.
- 56. Tiippana E, Bachmann M, Kalso E, Pere P. Effect of paracetamol and coxib with or without dexamethasone after laparoscopic cholecystectomy. Acta Anaesthesiol Scand 2008; 52: 673–680.
- 57. Moore RA, Wiffen PJ, Derry S, et al. Non‐prescription (OTC) oral analgesics for acute pain: an overview of Cochrane reviews. Cochrane Database Syst Rev 2015; CD010794.
- 58. Halila GC, dos Santos Czepula AI, Otuki MF, Correr CJ. Review of the efficacy and safety of over‐the-counter medicine. Braz J Pharm Sci 2015; 51: 403–414.





Abstract
Objective: To evaluate the efficacy and safety of paracetamol as an analgesic medication in a range of painful conditions.
Study design: Systematic review of systematic reviews of the analgesic effects of paracetamol in randomised, placebo‐controlled trials. Conduct of systematic reviews was assessed with AMSTAR‐2; confidence in effect estimates (quality of evidence) was assessed with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria.
Data sources: MEDLINE, EMBASE, PsycINFO, Cochrane Database of Systematic Reviews; systematic reviews published 1 January 2010 – 30 April 2020.
Data synthesis: We extracted pain and adverse events outcomes from 36 systematic reviews that assessed the efficacy of paracetamol in 44 painful conditions. Continuous pain outcomes were expressed as mean differences (MDs; standardised 0–10‐point scale); dichotomous outcomes were expressed as risk ratios (RRs). There is high quality evidence that paracetamol provides modest pain relief for people with knee or hip osteoarthritis (MD, –0.3 points; 95% CI, –0.6 to –0.1 points) and after craniotomy (MD, –0.8 points; 95% CI, –1.4 to –0.2 points); there is moderate quality evidence for its efficacy in tension‐type headache (pain‐free at 2 hours: RR, 1.3; 95% CI, 1.1–1.4) and perineal pain soon after childbirth (patients experiencing 50% pain relief: RR, 2.4; 95% CI, 1.5–3.8). There is high quality evidence that paracetamol is not effective for relieving acute low back pain (MD, 0.2 points; 95% CI, –0.1 to 0.4 points). Evidence regarding efficacy in other conditions was of low or very low quality. Frequency of adverse events was generally similar for people receiving placebo or paracetamol, except that transient elevation of blood liver enzyme levels was more frequent during repeated administration of paracetamol to patients with spinal pain (RR, 3.8; 95% CI, 1.9–7.4).
Conclusions: For most conditions, evidence regarding the effectiveness of paracetamol is insufficient for drawing firm conclusions. Evidence for its efficacy in four conditions was moderate to strong, and there is strong evidence that paracetamol is not effective for reducing acute low back pain. Investigations that evaluate more typical dosing regimens are required.
PROSPERO registration: CRD42015029282 (prospective).