Elevated serum angiotensin‐converting enzyme (ACE) activity, a biomarker for epithelioid granuloma, has a supportive role in the diagnosis and management of sarcoidosis,1 although in population‐based studies its diagnostic usefulness is modest, with positive and negative predictive values of 25.4% and 89.9% respectively.2 Further, elevated ACE activity is non‐specific; it is also found in people with tuberculous and other infectious granulomata, liver disease, lymphoma, diabetes, or hyperthyroidism, and also as a benign familial condition. However, elevated ACE activity can facilitate some clinical decisions, including the diagnosis of Löfgren syndrome or adults with uveitis.1,3
Serum ACE can be assessed by measuring its enzymatic activity or its protein concentration. Most Australian pathology laboratories measure ACE activity, which is predictably inhibited by ACE inhibitor (ACEI) drugs commonly prescribed for people with high blood pressure,4,5 whereas ACE protein level is not affected by these agents.
In this study, we investigated the prevalence of ACEI influencing ACE activity results; for cases of markedly elevated ACE, we also evaluated the clinical performance of the two ACE measures with respect to sarcoidosis. In a preliminary evaluation, all discrepant paired results (high mass with low activity) were for patients using ACEIs at the time of sample collection. Between January 2017 and February 2019, we measured ACE activity and protein concentration in parallel; all test requests were initiated by clinicians as part of routine clinical care. Formal ethics approval was not required for collecting and analysing data to assess the quality of routine care. Further details of the study design and laboratory methods are included in the online Supporting Information.
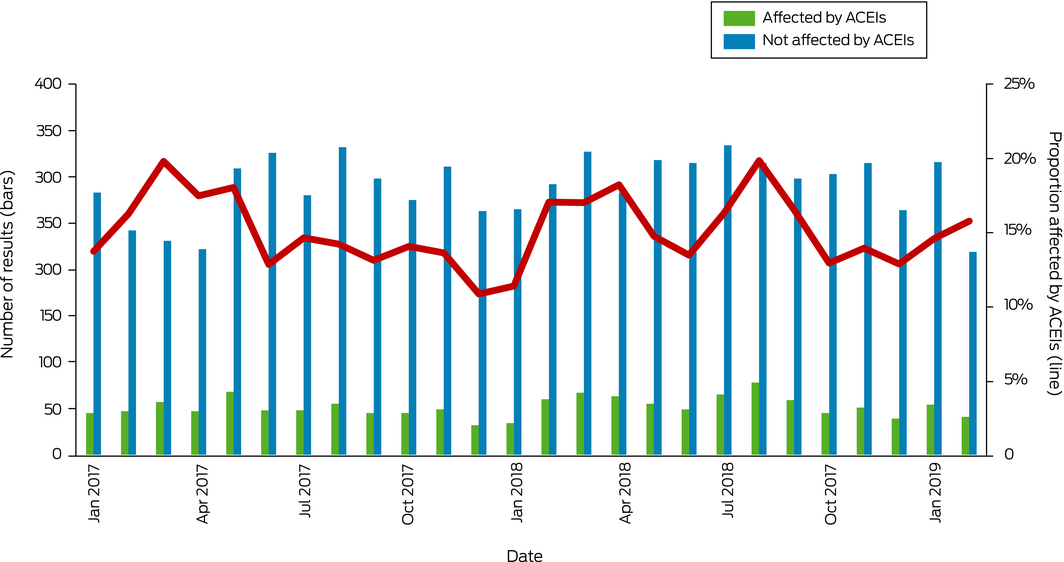
A total of 8882 paired test results were retrieved from the Pathology Queensland database for 4206 women (median age, 53.3 years; interquartile range [IQR], 36.0–65.6 years) and 4014 men (median age, 55.2 years; IQR, 42.2–67.3 years). Two discrete populations were evident in the scatterplot of paired results; for 1346 pairs (15.2%; 95% CI, 14.4–15.9%; green in Box 1), ACE activity was low relative to ACE protein, pathognomonic of ACEI interference. The upper reference limits for the two tests and the regression line for samples not affected by ACEIs nearly intersected, suggesting the general biologic equivalence of the two analytic methods and that the discordant results were not attributable to mismatched reference limits (Box 1). The correlation of values for the unaffected samples was moderate (R2 = 0.71) and the differences between the methods greater than predicted by their variances (Supporting Information, figure), indicating that the assays were not interchangeable. The monthly rate of ACEI interference was fairly consistent throughout the study period, despite comments to requesting physicians about the discrepancy between activity and protein levels included in pathology laboratory reports (Box 2).
Of the 50 patients with high ACE protein levels (more than 300 μg/L) and ACE activity below the upper reference limit (70 IU/L), 27 (54%; 95% CI, 40–67%) had sarcoidosis (including 16 with ACE activity below the lower reference limit of 20 IU/L). In contrast, four of 16 people (25%; 95% CI, 10–50%) with high ACE activity (greater than 100 IU/L) and ACE protein within the reference interval had sarcoidosis. From a diagnostic perspective, ACEIs erode the negative predictive value of ACE activity, the most useful characteristic of this biomarker (Box 1; Supporting Information, table).
Given that ACEI therapy interferes with ACE activity assessment, we recommend measuring ACE protein in routine practice, with the added benefit of convenience and safety of uninterrupted therapy for people taking ACEIs. The lack of influence of laboratory comments on testing behaviour is disappointing, but perhaps unsurprising given the information overload typical of modern medicine.6
Box 1 – Effect of angiotensin‐converting enzyme inhibitor (ACEI) therapy on serum ACE activity: scatterplot of paired ACE activity and protein assay results
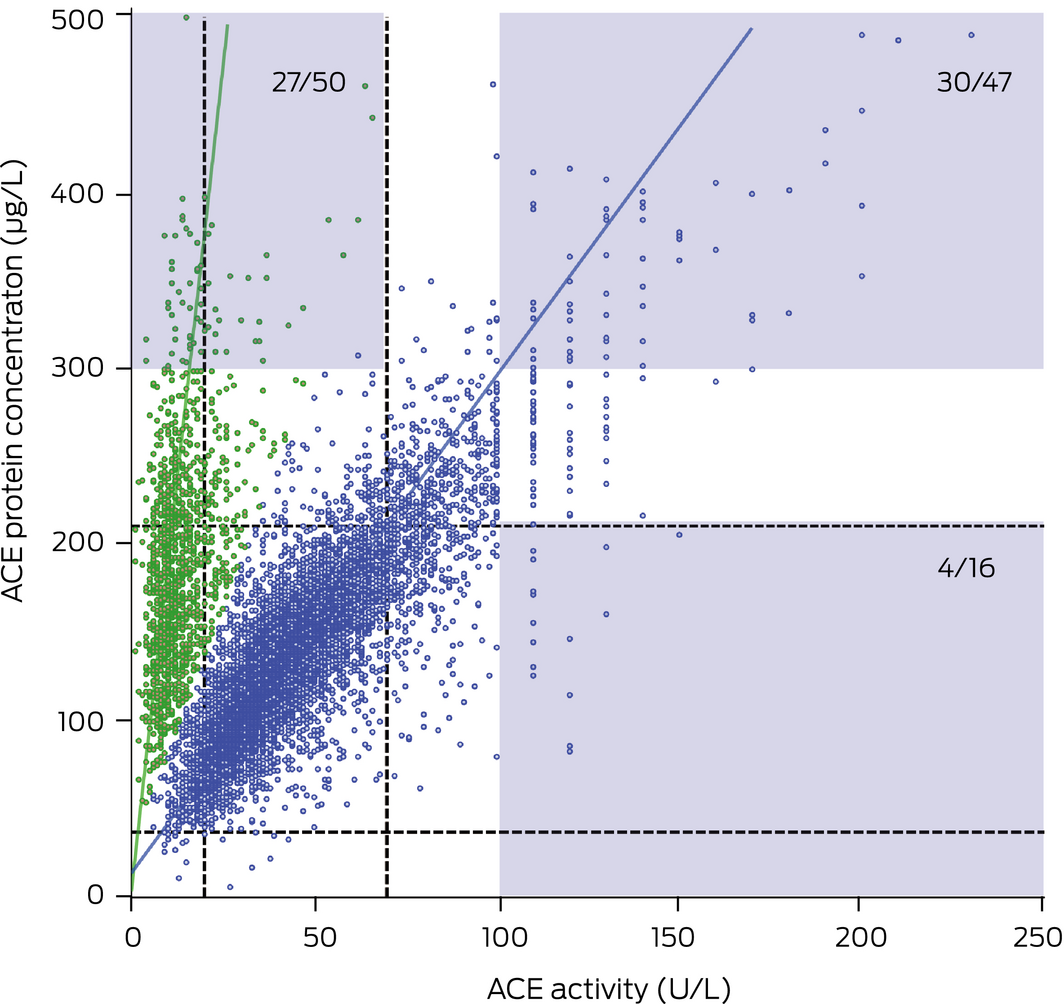
Pathology test reference intervals are indicated by the dotted lines. The shaded areas indicate result pairs included in the clinical audit (numbers of patients with sarcoidosis/total number audited). Blue: ACE activity not affected by ACEI therapy; 7536 samples, R2 = 0.71. Green: ACE activity affected by ACEI therapy; 1346 samples, R2 = 0.21.
Received 23 October 2019, accepted 11 February 2020
- 1. Ahmadzai H, Huang S, Steinfort C, et al. Sarcoidosis: a state of the art review from the Thoracic Society of Australia and New Zealand. Med J Aust 2018; 208: 499–504. https://www.mja.com.au/journal/2018/208/11/sarcoidosis-state-art-review-thoracic-society-australia-and-new-zealand.
- 2. Ungprasert P, Carmona EM, Crowson CS, Matteson EL. Diagnostic utility of angiotensin‐converting enzyme in sarcoidosis: a population‐based study. Lung 2016; 194: 91–95.
- 3. Niederer RL, Al‐Janabi A, Lightman SL, Tomkins‐Netzer O. Serum angiotensin‐converting enzyme has a high negative predictive value in the investigation for systemic sarcoidosis. Am J Opthtalmol 2018; 194: 82–87.
- 4. Lieberman J, Zakria F. Effect of captopril and enalapril medication on the serum ACE test for sarcoidosis. Sarcoidosis 1989; 6: 118–123.
- 5. Struthers AD, Anderson G, MacFadyen RJ, et al. Non‐adherence with ACE‐inhibitor treatment is common in heart failure and can be detected by routine serum ACE activity assays. Heart 1999; 82: 584–588.
- 6. Singh H, Spitzmueller C, Petersen NJ, et al. Information overload and missed test results in electronic health record‐based settings. JAMA Intern Med 2013; 173: 702–703.





No relevant disclosures.