The known: The Australian Institute of Health and Welfare (AIHW), like many cancer registries, calculates cumulative risk from cross‐sectional data to estimate lifetime risks of cancer diagnosis and mortality. Its method assumes there are no competing causes of death, which may lead to overestimation of lifetime risk.
The new: Adjusting for competing mortality reduced the estimated lifetime risks of cancer diagnosis and cancer‐specific mortality for the five most common cancers in Australia.
The implication: Estimates of cancer‐specific risk are widely circulated in awareness campaigns and for promoting early detection activities. Adjusted estimates would be more appropriate for informing choices by individuals.
Cancer (46 307 deaths in 20161) and coronary heart disease (19 077 deaths in 20162) are the leading causes of death in Australia. An estimated 470 new cancers were diagnosed and 161 cancer deaths recorded per 100 000 Australian adults in 2017; the five most frequently diagnosed cancers were breast cancer, colorectal cancer, prostate cancer, melanoma of the skin, and lung cancer.3 State‐based registries record and submit cancer incidence and mortality data to the Australian Institute of Health and Welfare (AIHW), which compiles these data annually for the Australian Cancer Database. The International Agency for Research on Cancer grades the quality of these data as “A”, the highest level on their scale.4 AIHW Australian Cancer Database reports are an important source of information on cancer epidemiology and guide initiatives for improving cancer prevention, screening, diagnosis, and treatment at the local, state, and national levels.5
The AIHW calculates cumulative risk by using cross‐sectional data to estimate the lifetime risks of cancer diagnoses. These risk estimates are important, as they are freely accessible to the public, clinicians, and organisations that employ them for a range of purposes. The AIHW first calculates the risk of being diagnosed with a particular cancer for each 5‐year age group interval, conditional on surviving to the beginning of that interval; these risks are then summed to obtain an overall estimate of the lifetime probability of cancer.3,6 This method overestimates the lifetime probability of cancer when compared with methods that account for competing mortality risk associated with non‐cancer conditions.7,8,9
We therefore hypothesised that the AIHW method overestimates lifetime cancer risks in Australia. In this study, we estimated the lifetime risks of cancer diagnosis and death for the five most common cancers in Australia, adjusted for competing mortality, and compared these estimates with the lifetime risks published by the AIHW.
Methods
We employed DevCan 6.7.5, freely available statistics software provided by the United States National Cancer Institute (https://surveillance.cancer.gov/devcan), to estimate the lifetime risks of diagnosis and mortality for breast cancer, prostate cancer, colorectal cancer, melanoma of the skin, and lung cancer, adjusted for competing mortality. DevCan 6.7.5 applies a lifetable approach to analysing cross‐sectional counts (or rates) of cancer‐specific incidence, cancer‐specific deaths, all‐cause deaths, and population numbers for sex and age group intervals from birth to the final years of life. The program estimates the probability of being diagnosed with a specific cancer for each age interval, conditional on being alive and cancer‐free at the beginning of the interval, in a unified competing risks framework.10 We described the statistical methods applied by the software in greater detail in our article on the estimated lifetime risk of prostate cancer overdiagnosis in Australia.11
Sex‐ and age‐specific cancer incidence and mortality and all‐cause mortality counts for the calendar years 1982–2013 were obtained from the AIHW.12,13,14,15,16,17 We extracted data for invasive cancer only and generated life tables to calculate the cumulative risks of diagnosis and death for each cancer for each calendar year, to age 85 years (the upper age limit for calculating lifetime risk used by the AIHW). We estimated lifetime risk adjusted for competing mortality as a percentage risk. For colorectal cancer, lung cancer, and melanoma, we estimated risks separately for women and men. We then compared our estimates with the estimates published by the AIHW. One of two authors (ACB, KL) undertook the calculations for a specific cancer type, and the other independently estimated the corresponding lifetime risks for the years 1985, 1990, 1995, 2000, 2005, and 2010 as a cross‐check.
Ethics approval
Approval by a Human Research Ethics Committee was not required for our analysis of publicly available aggregated population data.
Results
For all cancers and years examined, the published AIHW lifetime risks of cancer diagnosis (Box 1; Supporting Information, tables 1–5) and mortality (Box 1) were higher than our calculated lifetime risks, adjusted for competing mortality.
Invasive breast cancer (women)
Both the AIHW estimates and our adjusted estimates of lifetime risk of diagnosis increased markedly from 1993, corresponding to the introduction of the national mammography program in Australia. The difference between the AIHW estimate and our adjusted estimate declined from 1.5 percentage points (1982) to 0.6 percentage point (2013), and the difference between AIHW and adjusted risk of mortality estimates declined from 0.8 percentage point (1982) to 0.4 percentage point (2013) (Box 1, Box 2).
Prostate cancer (men)
Both the AIHW estimates and our adjusted estimates of lifetime risk of diagnosis increased markedly during the early 1990s, corresponding to the introduction of the prostate‐specific antigen (PSA) test. The difference between the AIHW estimate and our adjusted estimate was the largest for any of the cancers examined, but declined from 6.4 percentage points in 1982 to 2.5 percentage points in 2013. The differences between mortality estimates were also the greatest for any cancer, but declined from 3.0 percentage points (1982) to 0.9 percentage point (2013) (Box 1, Box 3).
Colorectal cancer (men and women)
The lifetime risks of colorectal cancer diagnoses remained steady for both men and women between 1982 and 2013. The differences between the AIHW and our competing mortality‐adjusted estimates declined from 4.0 percentage points (1982) to 2.0 percentage points (2013) for men, and from 1.8 percentage points (1982) to 0.9 percentage point (2013) for women. Differences between the estimates of lifetime mortality risk also declined, from 2.5 percentage points (1982) to 0.7 percentage point (2013) for men, and from 1.1 percentage points (1982) to 0.3 percentage point (2013) for women (Box 1, Box 4).
Invasive melanoma of the skin (men and women)
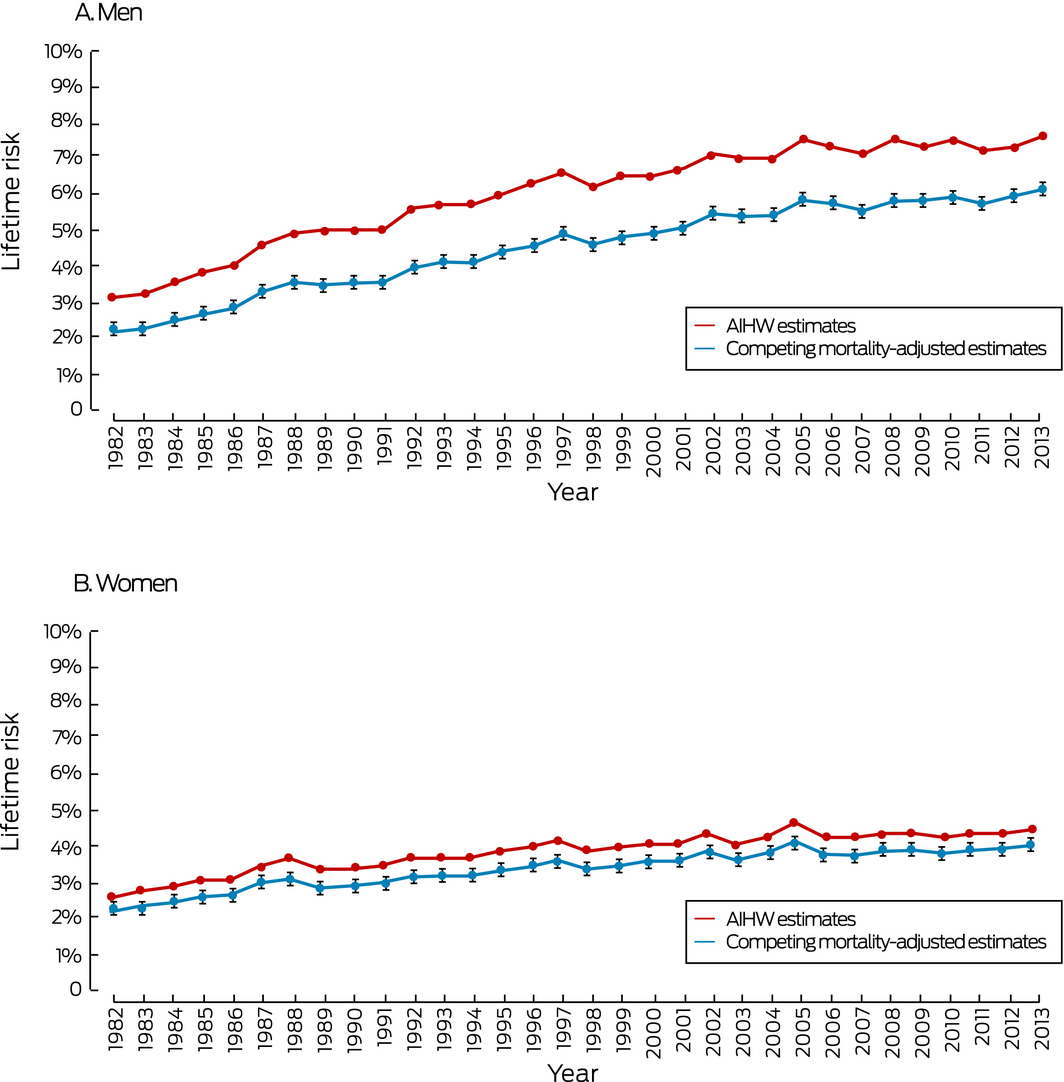
The lifetime risks of a melanoma diagnosis increased for both men and women between 1982 and 2013. The differences between the AIHW estimate and our adjusted estimate of lifetime risk of melanoma diagnosis were the smallest for any of the cancers examined, and remained steady over time: for men, 1.0 percentage point in 1982, 1.1 percentage point in 2013; for women, 0.4 percentage point in 1982, 0.4 percentage point in 2013. The differences in estimates of lifetime mortality risk were also the smallest for any cancer and steady over time: for men, 0.3 percentage point in 1982, 0.4 percentage point in 2013; for women, 0.1 percentage point in 1982, 0.0% in 2013 (Box 1, Box 5).
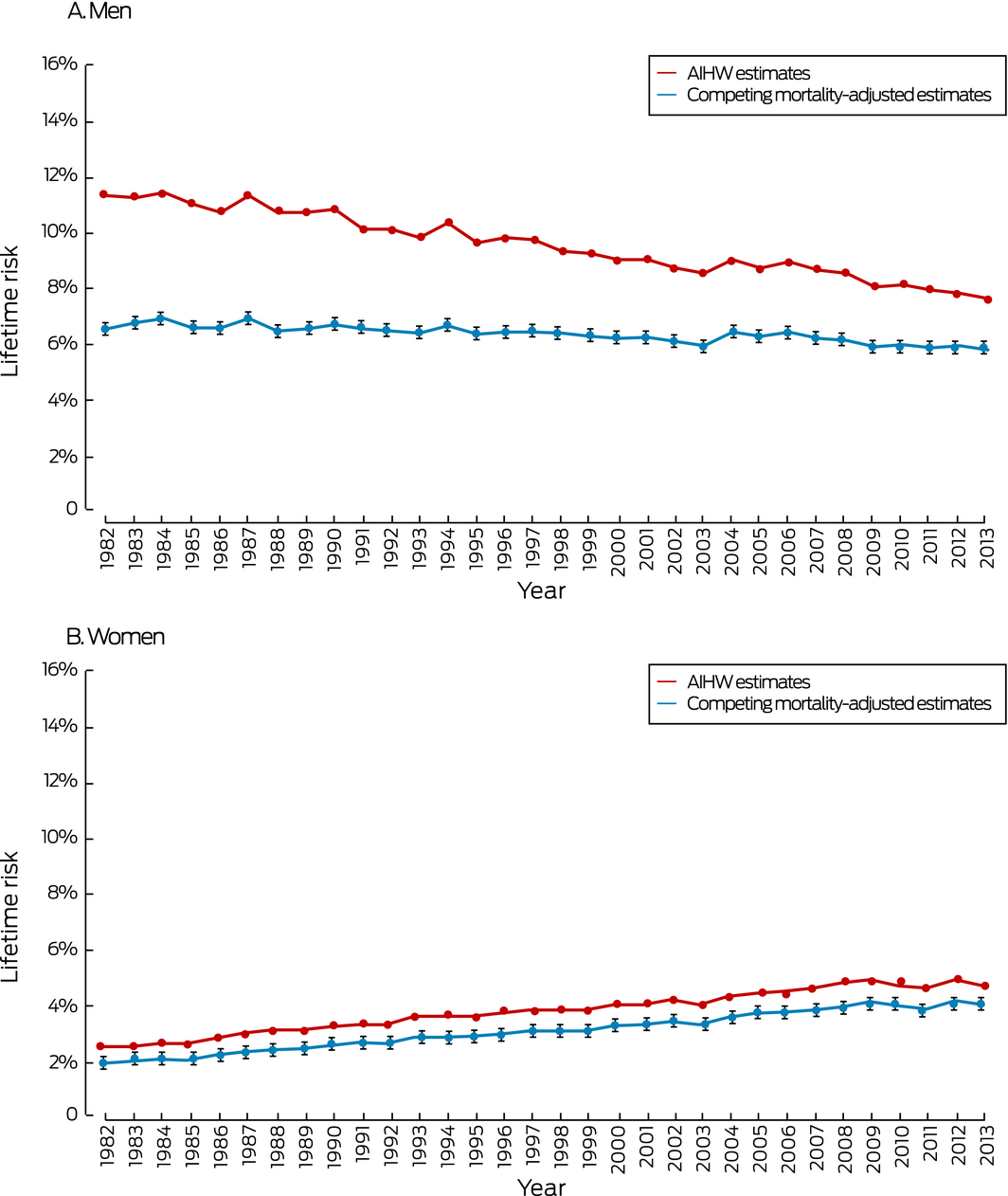
Lung cancer (men and women)
The lifetime risks for a lung cancer diagnosis increased for women but declined for men between 1982 and 2013. For men, the difference between the AIHW estimate and our adjusted estimate of lifetime risk of diagnosis declined from 4.8 percentage points (1982) to 1.8 percentage points (2013); the difference between the estimates for lifetime mortality risk declined from 4.9 percentage points (1982) to 1.6 percentage points (2013). For women, the differences in the estimates of lifetime risk of diagnosis (0.6 percentage point in 1982, 0.7 percentage point in 2013) and of mortality (0.5 percentage point in 1982, 0.5 percentage point in 2013) were steady across time (Box 1, Box 6).
Discussion
We found that AIHW estimates of the lifetime risks of diagnosis of or death from breast cancer, prostate cancer, colorectal cancer, melanoma of the skin, and lung cancer in Australia during 1982–2013 were consistently higher than estimates adjusted for competing mortality. The AIHW estimates were sometimes substantially higher; for example, the AIHW‐estimated lifetime risk for prostate cancer in 1993 was 22.5%, the competing mortality‐adjusted risk 14.4%. In 2013, the differences between lifetime risk of diagnosis estimates ranged from 0.4 percentage point for melanoma in women to 2.5 percentage points for prostate cancer in men.
For cancers for which the differences in estimated lifetime risk of a diagnosis exceeded 1% in 1982 (breast, prostate, and colorectal cancer, and lung cancer in men), the differences declined over time. This finding may be explained by falling competing mortality from cardiovascular disease.18 For cancers for which the differences were 1.0 percentage point or less in 1982 (lung cancer in women, melanoma), the differences changed little. The differences in estimated lifetime risks of diagnosis and mortality were consistently smaller for women than men, which may be explained by greater competing mortality risk for men from other causes, such as cardiovascular disease.19
We have reported estimates of lifetime risk as percentage risks, but health agencies and other organisations often communicate lifetime risks to the public as a natural frequency (ie, “one in x chance”). For example, Cancer Australia reports the current lifetime risk of prostate cancer for Australian men (to age 85) as 1 in 6,20 while the organisation “ManUp! Australia” describes it as 1 in 5.21 Risk communication studies suggest that this approach is not ideal for communicating risk; people have a clearer perception of risk when the denominator remains constant (eg, 1 in 10 v 2 in 10) rather than the numerator (eg, 1 in 10 v 1 in 5).22 The AIHW publishes lifetime risk estimates both as natural frequencies and as percentage risks, and both formats are affected by correction or non‐correction for competing risk; for example, the AIHW estimate for the lifetime risk of a prostate cancer diagnosis in 1993 was 1 in 4, while the risk after adjusting for competing mortality was 1 in 7.
Differences between AIHW and adjusted estimates of lifetime risk of cancer‐specific risk and mortality will not be eliminated if the AIHW estimates are not adjusted for competing mortality. Although such adjustment is clearly useful, it relies upon the availability of good quality administrative data to estimate competing mortality risks; these data are available in many high income countries, including Australia, but they are not available for all populations. Unlike the current AIHW approach, the DevCan method requires additional data, such as cancer‐specific and all‐cause mortality, and this adds further complexity to calculating lifetime risk. Finally, by adjusting for competing mortality, it may become more difficult to infer the extent to which observed changes in cancer‐specific risk are attributable to changes in risk factor prevalence, as opposed to variations in life expectancy resulting from changes in non‐cancer causes of mortality.8 This problem, however, is not relevant to the primary purpose of lifetime risk estimates, which is to provide risk information to the Australian public, clinicians, and policymakers, rather than to explore the underlying causes of changes in risk.
Our results indicate that cancer agencies, including the AIHW, may overestimate the risks of people being diagnosed with or dying from particular cancers. We are, not, however, criticising the AIHW. The method used by the AIHW is employed by many cancer registries; further, software that facilitates accounting for competing risk was not available when AIHW began calculating lifetime risks. DevCan has since become freely available from the US National Cancer Institute, rendering accounting for competing mortality more convenient. DevCan software is employed by American and Canadian agencies for calculating lifetime cancer risks,23 and a different method is applied in the United Kingdom for the same purpose.24
As lifetime risk estimates are widely cited in health promotion campaigns, they may cause public misperceptions of the risk of a cancer diagnosis or death. For example, lifetime cancer risk is used to increase awareness and to promote screening and other early detection activities by the Cancer Council Pink Ribbon campaign (breast and gynaecological cancers; https://www.pinkribbon.com.au), the Bowel Cancer Australia Red Apple Day (colorectal cancer; https://www.bowelcanceraustralia.org/red-apple-day), and the Prostate Cancer Foundation of Australia's Get Checked Initiative (https://www.prostate.org.au/get-checked). Overestimating the lifetime risk of a cancer may distort an individual's perception of the potential benefits and harms of screening, leading them to undergo testing they may not otherwise have chosen. It is therefore important that cancer agencies consider the impact of their choice of statistical methods.
Conclusion
Our findings suggest that the method currently used by the AIHW to estimate the lifetime risks of diagnosis and death associated with the five most common cancers is likely to overestimate these risks. Australian agencies should consider adopting methods for adjusting for competing mortality when estimating lifetime risks, as currently employed in North America and the United Kingdom, to increase the accuracy of their estimates.
Box 1 – Estimated lifetime risks of cancer diagnosis and mortality, Australia, 1982–2013
|
|
Lifetime risk of diagnosis |
Lifetime risk of mortality |
|||||||||||||
|
AIHW estimate |
Competing mortality‐adjusted estimate (95% CI) |
AIHW estimate |
Competing mortality‐adjusted estimate (95% CI) |
||||||||||||
|
|
|||||||||||||||
|
Invasive breast cancer (women) |
|
|
|
||||||||||||
|
1982 |
8.5% |
7.0% (6.8–7.2%) |
3.4% |
2.6% (2.5–2.8%) |
|||||||||||
|
1993 |
10.9% |
9.6% (9.4–9.9%) |
3.6% |
2.9% (2.8–3.0%) |
|||||||||||
|
2003 |
11.8% |
10.9% (10.7–11.1%) |
2.9% |
2.5% (2.4–2.6%) |
|||||||||||
|
2013 |
12.7% |
12.1% (11.9–12.3%) |
2.5% |
2.1% (2.0–2.2%) |
|||||||||||
|
Prostate cancer (men) |
|
|
|
|
|||||||||||
|
1982 |
11.7% |
5.3% (5.2–5.5%) |
5.0% |
2.0% (1.9–2.1%) |
|||||||||||
|
1993 |
22.5% |
14.4% (14.1–14.7%) |
6.0% |
3.1% (3.0–3.3%) |
|||||||||||
|
2003 |
19.5% |
15.0% (14.7–15.3%) |
4.6% |
2.9% (2.7–3.0%) |
|||||||||||
|
2013 |
18.7% |
16.2% (16.0–16.4%) |
3.3% |
2.4% (2.3–2.5%) |
|||||||||||
|
Colorectal cancer (men) |
|
|
|
|
|||||||||||
|
1982 |
8.8% |
4.8% (4.7–5.0%) |
5.0% |
2.5% (2.4–2.7%) |
|||||||||||
|
1993 |
9.7% |
6.4% (6.3–6.6%) |
4.6% |
2.8% (2.7–2.9%) |
|||||||||||
|
2003 |
10.0% |
7.3% (7.1–7.5%) |
3.7% |
2.5% (2.4–2.6%) |
|||||||||||
|
2013 |
9.0% |
7.0% (6.8–7.2%) |
2.6% |
1.9% (1.8–2.0%) |
|||||||||||
|
Colorectal cancer (women) |
|
|
|
||||||||||||
|
1982 |
6.6% |
4.8% (4.7–5.0%) |
3.5% |
2.4% (2.3–2.6%) |
|||||||||||
|
1993 |
6.8% |
5.4% (5.2–5.5%) |
3.1% |
2.3% (2.2–2.4%) |
|||||||||||
|
2003 |
7.0% |
5.8% (5.6–5.9%) |
2.3% |
1.8% (1.8–1.9%) |
|||||||||||
|
2013 |
6.4% |
5.5% (5.4–5.7%) |
1.7% |
1.4% (1.3–1.5%) |
|||||||||||
|
Invasive melanoma of skin (men) |
|
|
|
||||||||||||
|
1982 |
3.0% |
2.0% (1.9–2.1%) |
0.8% |
0.5% (0.4–0.5%) |
|||||||||||
|
1993 |
5.6% |
4.0% (3.9–4.1%) |
1.0% |
0.7% (0.6–0.7%) |
|||||||||||
|
2003 |
6.9% |
5.3% (5.1–5.4%) |
1.1% |
0.8% (0.7–0.8%) |
|||||||||||
|
2013 |
7.5% |
6.0% (5.9–6.2%) |
1.3% |
0.9% (0.9–1.0%) |
|||||||||||
|
Invasive melanoma of skin (women) |
|
|
|
||||||||||||
|
1982 |
2.5% |
2.1% (2.0–2.2%) |
0.4% |
0.3% (0.2–0.3%) |
|||||||||||
|
1993 |
3.6% |
3.1% (3.0–3.2%) |
0.4% |
0.3% (0.3–0.3%) |
|||||||||||
|
2003 |
4.0% |
3.6% (3.4–3.7%) |
0.5% |
0.4% (0.3–0.4%) |
|||||||||||
|
2013 |
4.4% |
4.0% (3.8–4.1%) |
0.4% |
0.4% (0.3–0.4%) |
|||||||||||
|
Lung cancer (men) |
|
|
|
|
|||||||||||
|
1982 |
11.3% |
6.5% (6.3–6.7%) |
10.8% |
5.9% (5.8–6.1%) |
|||||||||||
|
1993 |
9.8% |
6.4% (6.2–6.5%) |
9.0% |
5.7% (5.5–5.8%) |
|||||||||||
|
2003 |
8.5% |
5.9% (5.7–6.1%) |
7.2% |
5.0% (4.8–5.1%) |
|||||||||||
|
2013 |
7.6% |
5.8% (5.6–5.9%) |
5.9% |
4.3% (4.2–4.5%) |
|||||||||||
|
Lung cancer (women) |
|
|
|
|
|||||||||||
|
1982 |
2.4% |
1.8% (1.7–1.9%) |
2.0% |
1.5% (1.4–1.6%) |
|||||||||||
|
1993 |
3.5% |
2.7% (2.6–2.9%) |
2.9% |
2.3% (2.2–2.4%) |
|||||||||||
|
2003 |
3.9% |
3.2% (3.1–3.3%) |
3.2% |
2.6% (2.5–2.7%) |
|||||||||||
|
2013 |
4.6% |
3.9% (3.8–4.0%) |
3.2% |
2.7% (2.6–2.8%) |
|||||||||||
|
|
|||||||||||||||
|
AIHW = Australian Institute of Health and Welfare. |
|||||||||||||||
Box 2 – Lifetime risk of invasive breast cancer diagnosis for women, Australia, 1982–2013
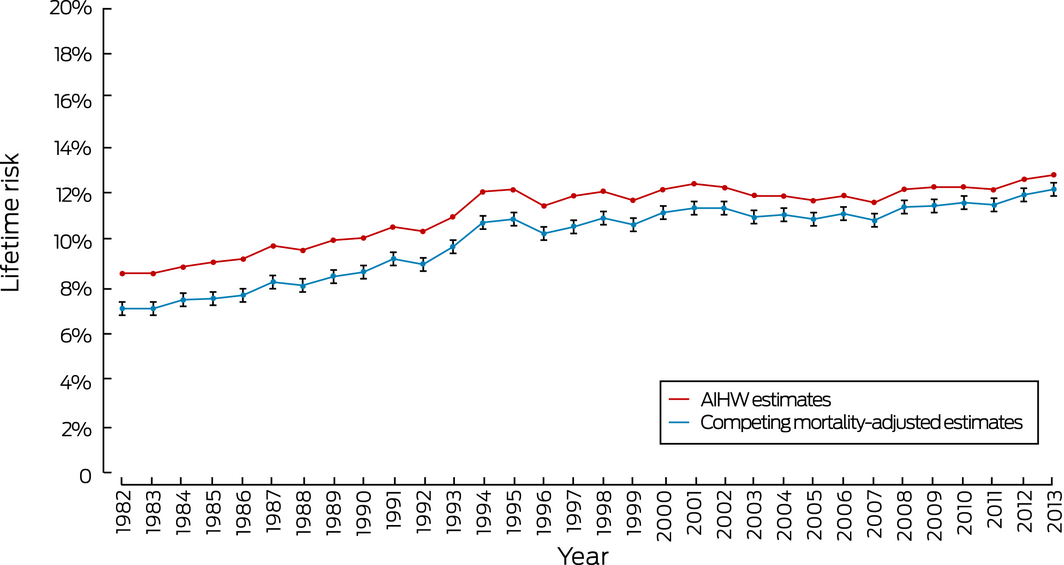
AIHW = Australian Institute of Health and Welfare. 95% confidence intervals are shown for the adjusted estimates.
Box 3 – Lifetime risk of prostate cancer diagnosis for men, Australia, 1982–2013
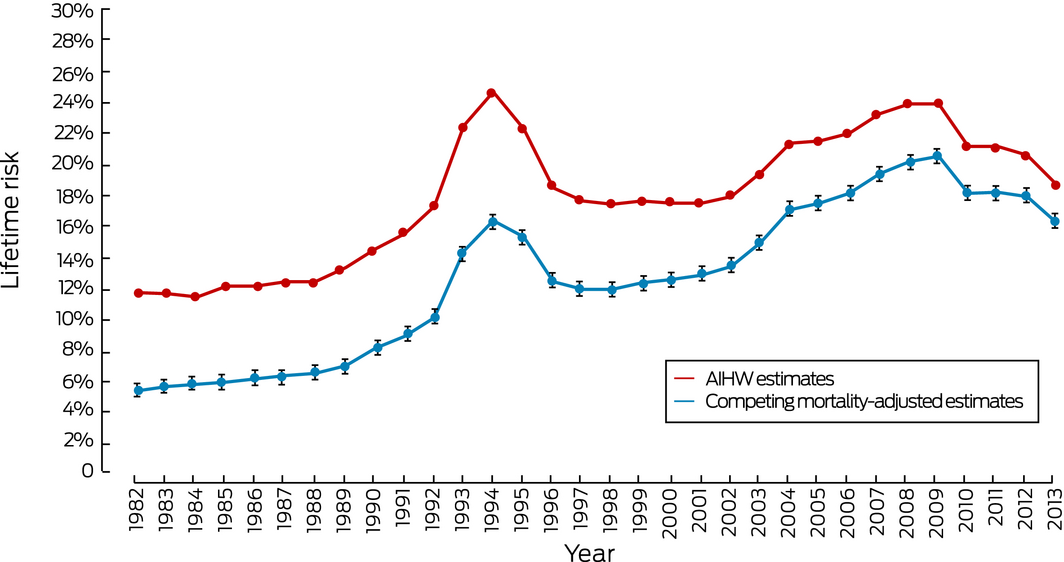
AIHW = Australian Institute of Health and Welfare. 95% confidence intervals are shown for the adjusted estimates.
Box 4 – Lifetime risk of colorectal cancer diagnosis, Australia, 1982–2013
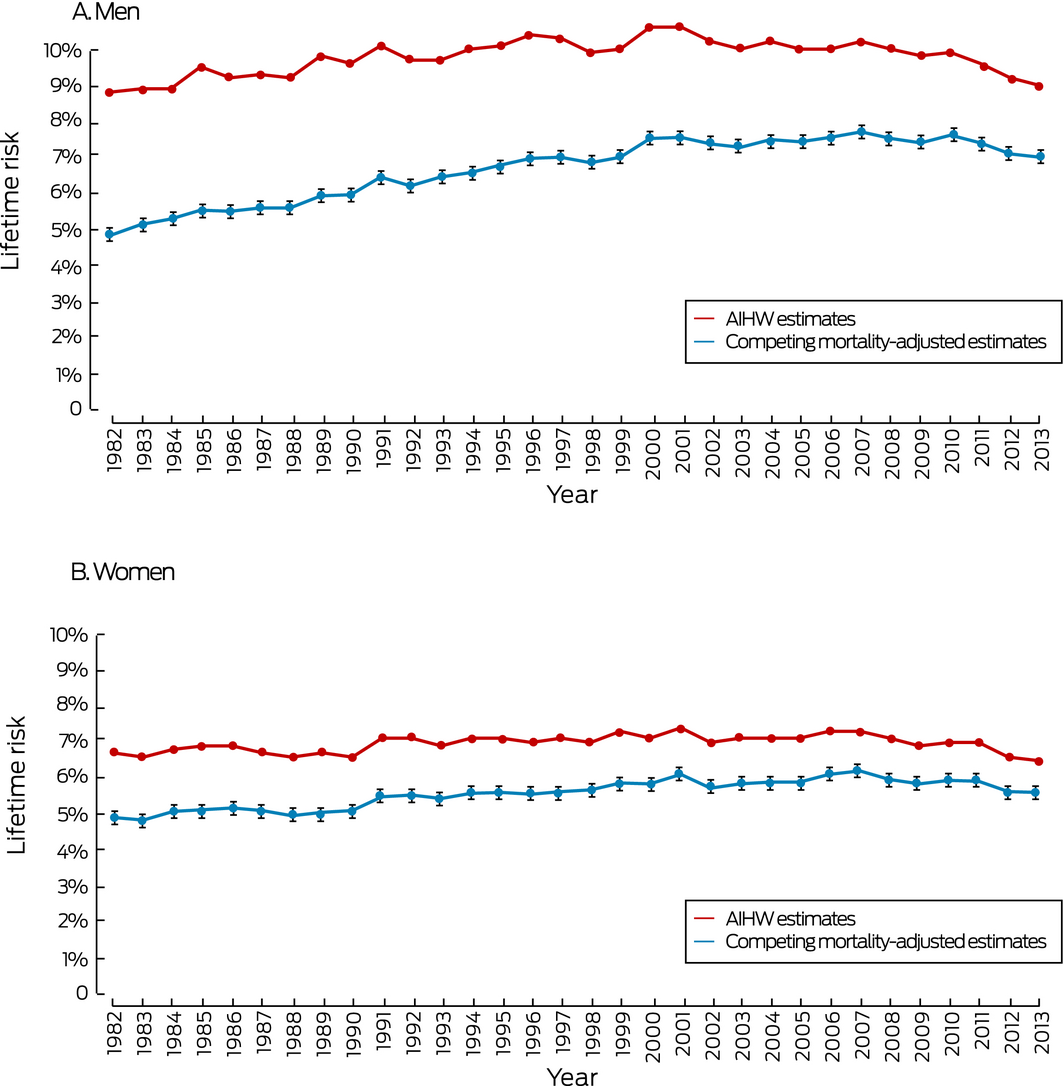
AIHW = Australian Institute of Health and Welfare. 95% confidence intervals are shown for the adjusted estimates.
Received 9 February 2019, accepted 24 June 2019
- Anthea C Bach1,2
- Kelvin SE Lo2,3
- Thanya Pathirana4,5
- Paul P Glasziou5
- Alexandra L Barratt6
- Mark A Jones5,7
- Katy JL Bell6
- 1 West Moreton Hospital and Health Service, Ipswich, QLD
- 2 Bond University, Gold Coast, QLD
- 3 Westmead Hospital, Sydney, NSW
- 4 Griffith University, Sunshine Coast, QLD
- 5 Institute for Evidence‐Based Healthcare, Bond University, Gold Coast, QLD
- 6 Sydney School of Public Health, University of Sydney, Sydney, NSW
- 7 University of Queensland, Brisbane, QLD
No relevant disclosures.
- 1. Australian Institute of Health and Welfare. General record of incidence of mortality workbooks: all neoplasms (ICD‐10 C00–D48), 1907–2016. Canberra: AIHW, 2016. https://www.aihw.gov.au/getmedia/15f69416-24aa-4255-827d-7b600827076b/grim0200-all-neoplasms.xlsx.aspx (viewed July 2017).
- 2. Australian Institute of Health and Welfare. General record of incidence of mortality workbooks: coronary heart disease (ICD‐10 I20–I25), 1940–2016. Canberra: AIHW, 2016. https://www.aihw.gov.au/getmedia/44b41fd9-3bc7-4c24-a9d9-ab6d85ed0c95/grim0909-coronary-heart-disease.xlsx.aspx (viewed Nov 2018).
- 3. Australian Institute of Health and Welfare. Cancer in Australia 2017 (Cat No. CAN 100; Cancer series no.101). Canberra: AIHW, 2017.
- 4. Australian Institute of Health and Welfare. Australian Cancer Database, 2014; quality statement. Dec 2017. http://meteor.aihw.gov.au/content/index.phtml/itemId/687104/pageDefinitionItemId/tag.MeteorPrinterFriendlyPage (viewed Nov 2018).
- 5. Roder D, Creighton N, Baker D, et al. Changing roles of population‐based cancer registries in Australia. Aust Health Rev 2015; 39: 425–428.
- 6. Boyle P, Parkin DM. Statistical methods for registries. In: Jensen OM, Parkin DM, MacLennan R, et al, editors. Cancer registration: principles and methods. Lyon: International Agency for Research on Cancer, 1991; pp. 147–150.
- 7. Schouten LJ, Straatman H, Kiemeney LA, Verbeek AL. Cancer incidence: life table risk versus cumulative risk. J Epidemiol Community Health 1994; 48: 596–600.
- 8. Satagopan JM, Ben‐Porat L, Berwick M, et al. A note on competing risks in survival data analysis. Br J Cancer 2004; 91: 1229–1235.
- 9. Tan KS, Eguchi T, Adusumilli PS. Competing risks and cancer‐specific mortality: why it matters. Oncotarget 2018; 9: 7272–7273.
- 10. Fay MP, Pfeiffer R, Cronin KA, et al. Age‐conditional probabilities of developing cancer. Stat Med 2003; 22: 1837–1848.
- 11. Pathirana TI, Hayen A, Doust J, et al. Lifetime risk of prostate cancer overdiagnosis in Australia: quantifying the risk of overdiagnosis associated with prostate cancer screening in Australia using a novel lifetime risk approach. BMJ Open 2019; 9: e022457.
- 12. Australian Institute of Health and Welfare. Australian cancer incidence and mortality books: breast cancer. Canberra: AIHW, 2017. https://www.aihw.gov.au/getmedia/53574d16-8a5f-4a90-a119-5dd716ee8dd2/breast-cancer-acim.xlsx.aspx (viewed July 2017).
- 13. Australian Institute of Health and Welfare. Australian cancer incidence and mortality books: colorectal cancer. Canberra: AIHW, 2017. https://www.aihw.gov.au/getmedia/64aee7ba-0633-4aa4-ab93-3555a96656b8/colorectal-cancer-acim.xlsx.aspx (viewed July 2017).
- 14. Australian Institute of Health and Welfare. Australian cancer incidence and mortality books: prostate cancer. Canberra: AIHW, 2017. https://www.aihw.gov.au/getmedia/51a8ac4c-416f-4ca7-a41f-faac7dd665c4/prostate-cancer-acim.xlsx.aspx (viewed July 2017).
- 15. Australian Institute of Health and Welfare. Australian cancer incidence and mortality books: melanoma of the skin. Canberra: AIHW, 2017. https://www.aihw.gov.au/getmedia/706696fd-63e5-4987-9031-8ecc7e954ae5/melanoma-of-the-skin-acim.xlsx.aspx (viewed July 2017).
- 16. Australian Institute of Health and Welfare. Australian cancer incidence and mortality books: lung cancer. Canberra: AIHW, 2017. https://www.aihw.gov.au/getmedia/abfee0a8-b736-4a5a-95dd-bfc9402b3121/lung-cancer-acim.xlsx.aspx (viewed July 2017).
- 17. Australian Institute of Health and Welfare. General record of incidence and mortality books 2013: all causes combined (AIHW Cat. No: PHE 217). Canberra: AIHW, 2015. https://www.aihw.gov.au/reports/life-expectancy-death/grim-books/contents/general-record-of-incidence-of-mortality-grim-books (viewed July 2017).
- 18. Australian Institute of Health and Welfare. Impact of falling cardiovascular disease death rates: deaths delayed and years of life extended (AIHW Cat. No. AUS113). Canberra: AIHW, 2009.
- 19. Dobson R. Men are more likely than women to die early. BMJ 2006; 333: 220.
- 20. Cancer Australia. Prostate cancer in Australia statistics [website]. Updated Aug 2019. https://prostate-cancer.canceraustralia.gov.au/statistics (viewed Aug 2019).
- 21. ManUp! Australia. Prostate cancer [website]. 2019. https://manupaustralia.org.au/prostate-cancer (viewed Aug 2019).
- 22. Trevena LJ, Zikmund‐Fisher BJ, Edwards A, et al. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak 2013; 13 (Suppl 2): S7.
- 23. Canadian Cancer Society's Advisory Committee on Cancer Statistics. Canadian cancer statistics 2017. June 2017. http://www.cancer.ca/~/media/cancer.ca/CW/cancer%20information/cancer%20101/Canadian%20cancer%20statistics/Canadian-Cancer-Statistics-2017-EN.pdf?la=en (viewed Dec 2018).
- 24. Cancer Research UK. Our calculations explained [website]. https://www.cancerresearchuk.org/health-professional/cancer-statistics/cancer-stats-explained/our-calculations-explained#heading-Eight (viewed Dec 2018).





Abstract
Objectives: To calculate lifetime risks of cancer diagnosis and cancer‐specific death, adjusted for competing mortality, and to compare these estimates with the corresponding risks published by the Australian Institute of Health and Welfare (AIHW).
Design, setting: Analysis of publicly available annual AIHW data on age‐specific cancer incidence and mortality — for breast cancer, colorectal cancer, prostate cancer, melanoma of the skin, and lung cancer — and all‐cause mortality in Australia, 1982–2013.
Outcome measures: Lifetime risks of cancer diagnosis and mortality (to age 85), adjusted for competing mortality.
Results: During 1982–2013, AIHW estimates were consistently higher than our competing mortality‐adjusted estimates of lifetime risks of diagnosis and death for all five cancers. Differences between AIHW and adjusted estimates declined with time for breast cancer, prostate cancer, colorectal cancer, and lung cancer (for men only), but remained steady for lung cancer (women only) and melanoma of the skin. In 2013, the respective estimated lifetime risks of diagnosis (AIHW and adjusted) were 12.7% and 12.1% for breast cancer, 18.7% and 16.2% for prostate cancer, 9.0% and 7.0% (men) and 6.4% and 5.5% (women) for colorectal cancer, 7.5% and 6.0% (men) and 4.4% and 4.0% (women) for melanoma of the skin, and 7.6% and 5.8% (men) and 4.5% and 3.9% (women) for lung cancer.
Conclusion: The method employed in Australia to calculate the lifetime risks of cancer diagnosis and mortality overestimates these risks, especially for men.