The known: Paracetamol is the drug most frequently taken in overdose in Australia. Large amounts can be purchased without prescription, whereas many European countries have reduced the incidence of paracetamol‐related harm by restricting pack sizes.
The new: Between 2007–08 and 2016–17, the annual number of paracetamol poisoning‐related hospital admissions increased by 3.8% per year, the number of cases of linked liver injury by 7.7% per year. Overdose size also increased between 2004 and 2017.
The implications: Public health measures that restrict the availability of paracetamol, such as reducing non‐prescription pack sizes, are needed to stem the increasing number of paracetamol overdoses.
Paracetamol is an effective analgesic and antipyretic. It is well tolerated and, at the recommended dose, is generally safe for healthy people. However, overdose or repeated supra‐therapeutic use can cause hepatotoxicity and death. In many countries, paracetamol is the drug most frequently involved in overdoses, and it is the most frequent cause of acute liver failure in the Western world.1 Australian poisons centres received 13 322 calls regarding paracetamol in 2015;2 United States poisons centres received more than 100 000 paracetamol‐related calls and recorded 313 deaths in 2016,3 while in the United Kingdom at least 80 000 people present to hospital with paracetamol overdoses each year, and there are 150–250 deaths.4
The gold standard treatment for paracetamol overdose is acetylcysteine. However, adverse outcomes in patients with massive paracetamol overdoses, despite early administration of acetylcysteine, have recently been reported.5,6 In the wake of increasing numbers of overdoses,7 liver transplantations, and deaths, and evidence that paracetamol overdose is often impulsive (taking medications already in the home),8 paracetamol pack sizes have been restricted in the UK since 19988 to 8 g for non‐pharmacy sales and to 16 g for pharmacy sales (formerly: 50 g). The approach appeared effective, as the number of large paracetamol‐related overdoses, liver unit admissions, and suicide deaths in England and Wales subsequently declined.9,10,11 However, these changes were not seen in Scotland,12 and the effectiveness of the measure has been questioned.13 In 2009, the availability of non‐prescription paracetamol was restricted in Germany to a maximum 10 g, and it can be purchased only in pharmacies.13 Most western European countries have similar restrictions.14
Paracetamol is the substance most frequently involved in overdoses in Australia.15 It is available outside pharmacies in packs of twenty 500 mg tablets (10 g), and from pharmacies as 100 × 500 mg (50 g) tablet packs and 96 × 665 mg (about 64 g) modified release (MR) tablet packs; there is no legal limit to the number of packs that can be purchased. Australian data on paracetamol overdose, including overdose size, liver injury, and deaths, are limited. We therefore examined the numbers of paracetamol overdose‐related hospital admissions and deaths in Australia since 2007–08, and the overdose size in intentional paracetamol overdoses since 2004.
Methods
Design and data sources
Our retrospective study focused on intentional paracetamol overdoses. Paracetamol overdose was defined as consuming a quantity that exceeded appropriate therapeutic levels, intentional overdose as knowingly consuming excessive amounts of single ingredient paracetamol preparations (eg, for self‐harm or manipulative purposes).
Demographic information and data on liver injury and in‐hospital deaths were obtained from the Australian Institute of Health and Welfare (AIHW) National Hospital Morbidity Database (NHMD), a national database of person‐level records for hospital admissions to public and private hospitals. The NHMD does not record the drug amounts ingested, so overdose size information was obtained from the New South Wales Poisons Information Centre (NSWPIC) database. NSWPIC is the largest poisons information centre in Australia, receiving about half the 205 000 poisons‐related calls made each year in Australia; it takes calls from health care professionals and members of the public in NSW (65% of calls) and interstate.16 As the NHMD captures death data only for in‐hospital deaths, these data were examined in conjunction with data from the National Coronial Information System (NCIS), the national database for all reportable deaths in Australia, managed by the Victorian Department of Justice and Community Safety.
These three sources provide complementary data that together deliver a detailed picture of paracetamol overdose in Australia. Paracetamol overdoses were identified as admissions in the NHMD with the relevant paracetamol poisoning code, exposures in the NSWPIC database coded with “paracetamol”, and deaths recorded in the NCIS attributable to paracetamol overdose after manual review.
NHMD hospital admissions data
NHMD data are collated annually and were available for the 10‐year period, 2007–08 to 2016–17. We searched the NHMD for cases with International Classification of Diseases, tenth revision, Australian modification (ICD‐10‐AM) codes T39.1 (poisoning by 4‐aminophenol derivatives; paracetamol is the only 4‐aminophenol derivative used as a drug in Australia), with or without code K71 (toxic liver disease) in the principal diagnosis field or in one of the nine additional diagnosis fields. Sex, age (5‐year age brackets), and the in‐hospital death flag were extracted. We identified exposure intent from external cause codes.
NSW Poisons Information Centre data
We searched the NSWPIC database for intentional paracetamol exposures during 2004–2017. Combination products (eg, paracetamol/codeine) were excluded because their pack sizes and access restrictions are different to those of paracetamol‐only products, but MR paracetamol was included. Overdoses involving multiple drugs were included if the paracetamol component was a single ingredient paracetamol product. Free text dose information was re‐coded as the number of tablets taken in the overdose; when a range was specified, the upper end of the range was selected.
NCIS deaths data
We searched the NCIS for cases from the period July 2007 to June 2017 and closed by September 2018 for which “paracet*” was recorded in the cause of death field (all levels) or “paracetamol” was listed as the parent drug of the pharmaceutical substance causing injury. As the coronial coding of drugs causing death is inconsistent (people often take several drugs at once, and the primary cause of death can be expressed in different ways), cases were manually reviewed. They were included if the death was attributed to paracetamol (history of overdose, elevated level with hepatotoxicity, or massive levels that could lead to coma). The free text of electronic reports was reviewed for the paracetamol product and amount taken. An additional search on 5 March 2019 captured cases closed since September 2018.
A significant overlap between deaths included in the NHMD and NCIS is probable, but the NHMD dataset did not include identifiers or other parameters that allowed matching to NCIS cases.
Statistical analysis
Changes in numbers of paracetamol poisonings in the NHMD and NSWPIC datasets were analysed by Poisson regression and negative binomial regression with a loglink function, with year as the independent variable and exposure count the outcome. Overdispersion of data was assessed with ratios of deviance to degrees of freedom and of Pearson χ2 to degrees of freedom; if the ratios exceeded 2 in the Poisson model, the data were deemed over‐dispersed, and negative binomial regression was employed.17 We also assessed fit with Bayesian and Akaike information criteria.
The number of tablets taken in overdoses (NSWPIC data) were summarised as medians with interquartile ranges (IQRs). Differences in the distribution of overdose sizes over time in the NSWPIC data were assessed in a Jonckheere–Terpstra test; the distributions of overdose size with immediate and modified release paracetamol preparations were compared in a Mann–Whitney test.
Analyses were performed in SAS 9.4 (SAS Institute) and R 3.4.1 (R Project for Statistical Computing) using the clinfun package. P < 0.05 was deemed statistically significant.
Ethics approval
The study was approved by the Sydney Children's Hospitals Network (reference, LNR/16/SCHN/44) and the Victorian Department of Justice Human Research Ethics Committee (reference, CF/15/18367).
Results
Paracetamol overdose‐related admissions
The NHMD included data for 95 668 admissions of patients with paracetamol poisonings (ICD‐10‐AM code T39.1) during the period 2007–08 to 2016–17. The annual number increased from 8147 in 2007–08 to 11 754 in 2016–17, an overall increase of 44.3% (3.8% per year; 95% confidence interval [CI], 3.2–4.6%; P < 0.001); the proportion of cases attributed to self‐harm consistently exceeded 75% (Box 1, A). Most patients were female (68 494, 71.6%); 27 168 were male (28.4%), and for six cases (< 0.01%) sex was not recorded. The most marked increases (in absolute numbers of cases) were for children aged 10–14 years (from 282 to 656 per year; 133% increase), adolescents (15–19 years; from 1842 to 2972; 61% increase), and young adults (20–24 [from 1164 to 1605; 38% increase] and 25–29 years [from 774 to 1091; 41% increase]); large relative increases were also recorded for older age groups (50–54 years, 64%; 60–64 years, 95%; 65–69 and 70–74 years, 160%; 85 years or older, 253%) (Box 2). T39.1 and K71 (toxic liver disease) diagnoses were both recorded for 1816 admitted patients; the proportion of cases attributed to self‐harm was about 56% (Box 1, B); the number increased from 116 in 2007–08 to 241 in 2016–17, an overall increase of 108% (7.7% per year; 95% CI, 6.0–9.5%; P < 0.001).
Paracetamol overdose size
The NSWPIC database included data for 22 997 cases of intentional overdose with paracetamol meeting our inclusion criteria. The annual number increased from 1263 in 2004 to 2235 in 2017, an overall increase of 77.0% (3.3% per year; 95% CI, 2.5–4.2%; P < 0.001) (Box 3, A). Most patients were female (16 181, 70.4%); 5644 (24.5%) were male, and sex was not recorded in 1172 cases (5.1%). Accurate age data were captured for 14 326 patients (62.3%); their median age was 18 years (IQR, 16–28 years). The median number of tablets taken per overdose increased from 15 (IQR, 10–24) in 2004 to 20 (IQR, 10–35) in 2017 (Jonckheere–Terpstra test of trend in median, P < 0.001) (Box 3, B).
MR paracetamol was taken in 1573 of the 22 997 overdoses, including 985 of 7797 paracetamol overdoses (12.6%) during 2014–2017. The annual number of overdoses with MR paracetamol increased from two in 2004 to 259 in 2017, an increase of 38.3% per year (95% CI, 29.9–47.3%; P < 0.001) (Box 3, A). Overdoses with MR paracetamol were larger (median, 19 tablets; IQR, 10–24) than those with immediate release paracetamol (median, 16 tablets; IQR, 10–35; Mann–Whitney test, P < 0.001).
Paracetamol overdose deaths
The NHMD recorded 434 in‐hospital deaths with T39.1 diagnosis codes (annual mean, 43 deaths per year; range, 34–57 deaths per year). This included 126 deaths with both T39.1 and K71 codes (annual mean, 13 deaths per year; range, 7–18 deaths per year) (Box 4, A), including 79 women (63%). The number of deaths with both codes was highest in the 40–44‐year (22 deaths) and 50–55‐year age groups (19 deaths). Fifty‐one cases (40%) were the result of self‐harm, 40 cases (32%) were accidental, and intent was unclear in 35 cases (28%).
Paracetamol was recorded as a cause of death or as the substance causing injury in 3162 NCIS cases (in‐ and out‐of‐hospital deaths). This included 205 cases in which paracetamol was deemed the primary cause of death after case review (Box 4, B). The median age in these cases was 53 years (IQR, 41–66 years), and 131 deaths (64%) were of women; 69 deaths (34%) were out‐of‐hospital deaths. Modified release paracetamol was involved in 24 of the 72 cases for which the formulation type was documented, all since 2009. There were no temporal patterns in the numbers of deaths recorded by either database.
Discussion
We found that the annual number of paracetamol‐related hospital admissions (annual increase, 3.8%) and the incidence of paracetamol‐related liver injury (annual increase, 7.7%) grew more rapidly in Australia during 2007–08 to 2016–17 than the national population (mean annual increase, 2004–2017, 1.6%18), as did the number of paracetamol‐related calls to NSWPIC during 2004–2017 (3.3%). The number of paracetamol‐related deaths, however, remained fairly constant.
Most paracetamol overdoses involved women (about 70%); the median age of patients in the NSWPIC database was 18 years (IQR, 16–28 years). The median age in cases of fatal overdoses recorded in the NCIS was higher (53 years; IQR, 41–66 years), perhaps reflecting greater suicidal intent in overdoses by older people or the presence of comorbid conditions that increase the risk of liver injury. Admissions to hospital with paracetamol poisoning and liver injury increased at twice the rate of all paracetamol‐related admissions (7.7% v 3.3% per year). This is consistent with NSWPIC data that indicated a significant increase in overdose size and an increased proportion of overdoses with MR paracetamol, which was implicated in 9.5% of overdoses during 2009–2017 but in 33% of fatal overdoses during 2009–2017 for which the formulation was documented. The overall number of paracetamol‐related deaths was fairly consistent across the study period; improved treatment guidelines may explain the apparent drop in the case fatality rate.
Paracetamol pack sizes and availability differ markedly between countries. In Australia, sales of 20‐tablet packs (10 g) are unrestricted; larger packs (50 g) have been available in pharmacies under Schedule 2, which does not require a pharmacist to be involved in the sale. In the US,19 Canada,19 and Russia,14 paracetamol is available without restriction, including from non‐pharmacy retailers. In 2018, 14 of 21 surveyed European countries had pharmacy pack size restrictions (range, 8–30 g); most European countries do not permit non‐pharmacy sales, and the rates of poisons centre calls regarding paracetamol are lower in these states.14 A comparison of French and British data suggested that greater availability of paracetamol was associated with its increased use in overdoses and suicide.20
It is thought that reducing pack sizes would reduce overdose size in cases of impulsive overdose. In Australia, the most widely available pack size was reduced in September 2013 from 25 to 20 × 500 mg tablets (which still provides a potentially hepatotoxic amount), with no change in pharmacy pack sizes.21 According to our findings, this small change appears to have had little impact on overdose sizes, and more restrictive changes, as in the UK, may be appropriate.
In Australia, MR paracetamol is available only in one pack size (96 × 665 mg). We found that MR overdoses involved significantly more tablets than overdoses with standard paracetamol; as the tablets are also higher strength, the total amount of paracetamol taken was also higher (median, 12.6 g v 8 g). The pharmacokinetics of MR paracetamol overdoses are unpredictable; such overdoses are also more difficult to treat, and hepatotoxicity can ensue despite early administration of acetylcysteine.22 In response to concerns about the higher overdose risk, the European Medicines Authority decided in 2018 to suspend marketing of MR paracetamol in Europe, noting that its only advantage was that it could be taken three rather than four times per day.23 In Australia, the Therapeutic Goods Administration has recently announced plans to up‐schedule MR paracetamol from Schedule 2 to Schedule 3 (pharmacist only).24
Limitations
The NHMD captures all Australian public and private hospital admissions, but does not include data for patients who were not admitted to hospital, but were, for instance, sent home from the emergency department. Patients with data in the NHMD were probably admitted because acetylcysteine treatment was deemed necessary, and thus constituted a group with larger overdoses or more severe poisonings. We examined admissions and deaths for which both paracetamol poisoning and toxic liver disease were reported; the liver injury can probably be attributed in these cases to paracetamol poisoning, but we cannot exclude the possibility of other causes.
NCIS data are limited by the interval before case closure (about 60% of 2017 cases were closed at the time of data extraction), so that our more recent figures must be interpreted with caution.
The NSWPIC is not a national database, but receives about 50% of PIC calls about poisoning in Australia,25 and its data therefore probably reflect the national situation. As NSWPIC cases are based on spontaneous reports of exposures, their numbers do not indicate the overall prevalence of paracetamol overdose; it is estimated that NSWPIC receives calls for about 25% of deliberate paracetamol overdoses in Australia (mean calls [2016–2017], 2092; intentional poisonings in NHMD [2016/17], 9356), or 50% of cases in its coverage area. Further, overdoses with combination products (eg, paracetamol/codeine, cough and cold preparations) were not included in the NSWPIC dataset. Hospitals with toxicology units or staffed by emergency physicians are less likely to contact the NSWPIC, and the availability of national guidelines for paracetamol overdose management may further reduce the need for calls. Conversely, uncertainty about how MR paracetamol overdoses should be managed may have increased the number of related calls and therefore our estimate of the proportion of overdoses linked with MR paracetamol. Further, call numbers to PICs may increase around the time of guideline changes or updates (2008 and 2015). Despite these limitations, the NSWPIC data could be disaggregated by overdose size and formulation, which is not possible with NHMD data and difficult with NCIS data.
Conclusion
The numbers of hospital admissions and cases of liver injury attributed to paracetamol overdose have increased in Australia since 2004, and the number and reported size of overdoses reported to the NSWPIC have also risen. Access restrictions, including reduced pack sizes, could reduce the harm caused by paracetamol overdoses in Australia, and should be considered, together with other policy changes for curbing this growing problem.
Box 1 – A. Hospital admissions of patients with paracetamol poisoning, 2007–08 to 2016–17. B. Hospital admissions of patients with paracetamol poisoning and toxic liver disease, 2007–08 to 2016–17
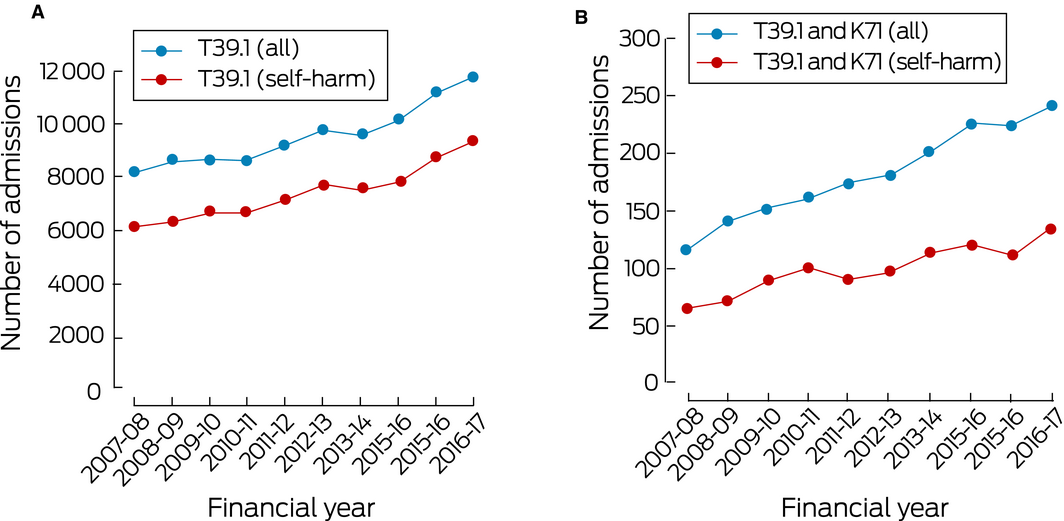
Source: National Hospital Morbidity Database.
Box 2 – Age distribution of people admitted to hospital with paracetamol poisoning, 2007–08 to 2016–17, by 5‐year age brackets
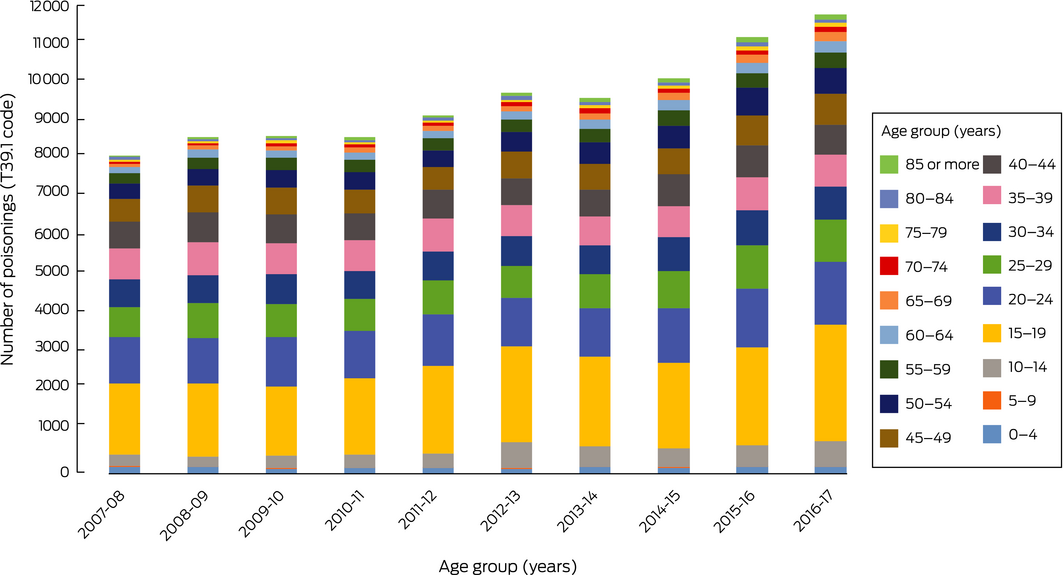
Source: National Hospital Morbidity Database.
Box 3 – A. Intentional overdose exposures with single ingredient paracetamol products, 2004–2017. B. Overdose sizes for single ingredient paracetamol products in cases for which size was recorded, 2004–2017
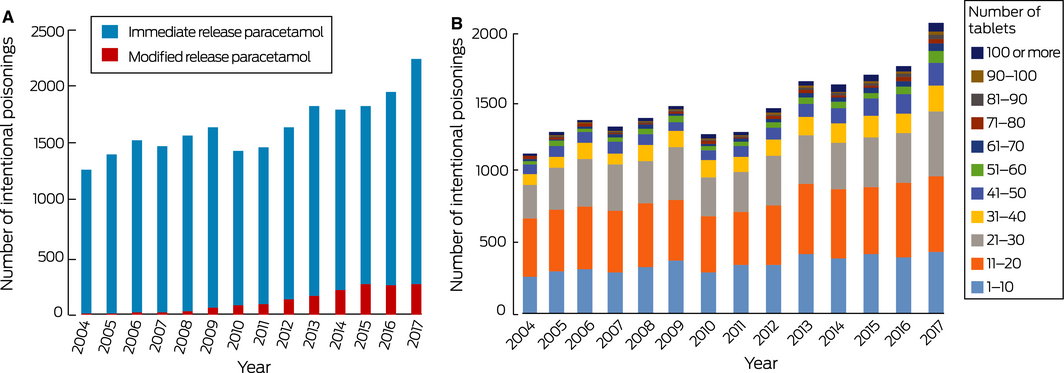
Source: New South Wales Poisons Information Centre. Data are depicted as proportions of all cases by year in the online Supporting Information.
Box 4 – A. In‐hospital deaths of patients with admission codings for both paracetamol poisoning and toxic liver disease, 2007–08 to 2016–17.* B. Paracetamol‐related deaths, 2007–08 to 2016–17, by formulation†
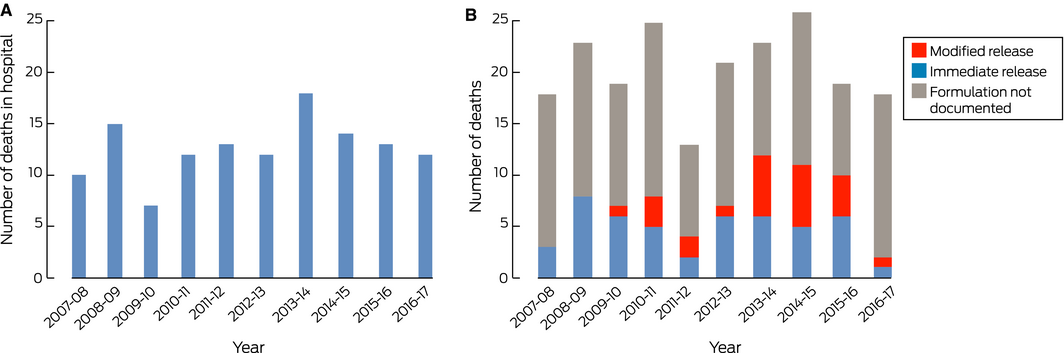
* Source: National Hospital Morbidity Database. † When recorded in case record; source: National Coronial Information System.
Received 11 April 2019, accepted 29 May 2019
- Rose Cairns1,2
- Jared A Brown1,3
- Claire E Wylie2
- Andrew H Dawson1,4
- Geoffrey K Isbister5,6
- Nicholas A Buckley1,2
- 1 NSW Poisons Information Centre, Children's Hospital at Westmead, Sydney, NSW
- 2 University of Sydney, Sydney, NSW
- 3 Centre for Big Data Research in Health, University of New South Wales, Sydney, NSW
- 4 Royal Prince Alfred Hospital, Sydney, NSW
- 5 University of Newcastle, Newcastle, NSW
- 6 Calvary Mater Newcastle, Newcastle, NSW
This study was supported by a National Health and Medical Research Council Program Grant (1055176). We acknowledge the Australian Institute of Health and Welfare for providing the National Hospital Morbidity Database data, and the National Coronial Information System (NCIS), managed by the Victorian Department of Justice and Community Safety, for providing the coronial data. We thank the staff of the New South Wales Poisons Information Centre for their contributions to the NSWPIC database.
No relevant disclosures.
- 1. Larson AM, Polson J, Fontana RJ, et al. Acetaminophen‐induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 2005; 42: 1364–1372.
- 2. Huynh A, Cairns R, Brown JA, et al. Patterns of poisoning exposure at different ages: the 2015 annual report of the Australian Poisons Information Centres. Med J Aust 2018; 209: 74–79. https://www.mja.com.au/journal/2018/209/2/patterns-poisoning-exposure-different-ages-2015-annual-report-australian-poisons.
- 3. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th annual report. Clin Toxicol 2017; 55: 1072–1252.
- 4. Bateman DN, Carroll R, Pettie J, et al. Effect of the UK's revised paracetamol poisoning management guidelines on admissions, adverse reactions and costs of treatment. Br J Clin Pharmacol 2014; 78: 610–618.
- 5. Cairney DG, Beckwith HKS, Al‐Hourani K, et al. Plasma paracetamol concentration at hospital presentation has a dose‐dependent relationship with liver injury despite prompt treatment with intravenous acetylcysteine. Clin Toxicol 2016; 54: 405–410.
- 6. Chiew AL, Isbister GK, Kirby KA, et al. Massive paracetamol overdose: an observational study of the effect of activated charcoal and increased acetylcysteine dose (ATOM‐2). Clin Toxicol 2017; 55: 1055–1065.
- 7. Hawton K, Fagg J, Simkin S, et al. Trends in deliberate self‐harm in Oxford, 1985–1995: implications for clinical services and the prevention of suicide. Br J Psychiatry 1995; 171: 556–560.
- 8. Hawton K, Bergen H, Simkin S, et al. Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyses. BMJ 2013; 346: 1–9.
- 9. Hawton K, Simkin S, Deeks J, et al. UK legislation on analgesic packs: before and after study of long term effect on poisonings. BMJ 2004; 329: 1076–1079.
- 10. Hawton K, Townsend E, Deeks J, et al. Effects of legislation restricting pack sizes of paracetamol and salicylate on self poisoning in the United Kingdom: before and after study. BMJ 2001; 322: 1–7.
- 11. Bernal W. changing patterns of causation and the use of transplantation in the United Kingdom. Semin Liver Dis 2003; 23: 227–236.
- 12. Bateman DN, Gorman DR, Bain M, et al. Legislation restricting paracetamol sales and patterns of self‐harm and death from paracetamol‐containing preparations in Scotland. Br J Clin Pharmacol 2006; 62: 573–581.
- 13. Bateman DN. Limiting paracetamol pack size: has it worked in the UK? Clin Toxicol 2009; 47: 536–541.
- 14. Morthorst BR, Erlangsen A, Nordentoft M, et al. Availability of paracetamol sold over the counter in Europe: a descriptive cross‐sectional international survey of pack size restriction. Basic Clin Pharmacol Toxicol 2018; 122: 643–649.
- 15. Chiew AL, Fountain JS, Graudins A, et al. Summary statement: new guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med J Aust 2015; 203: 215–218. https://www.mja.com.au/journal/2015/203/5/summary-statement-new-guidelines-management-paracetamol-poisoning-australia-and.
- 16. Cairns R, Brown J, Buckley N. The impact of codeine re‐scheduling on misuse: a retrospective review of calls to Australia's largest poisons centre. Addiction 2016; 111: 1848–1853.
- 17. Pedan A. Analysis of count data using the SAS system. Twenty‐Sixth Annual SAS Users Group International Conference, Long Beach (US), 22–25 Apr 2001; paper 247‐26. https://support.sas.com/resources/papers/proceedings/proceedings/sugi26/p247-26.pdf (viewed June 2019).
- 18. Australian Bureau of Statistics. 3105.0.65.001. Australian historical population statistics, 2016. Apr 2019. https://www.abs.gov.au/AUSSTATS/abs@.nsf/mf/3105.0.65.001 (viewed June 2019).
- 19. The Acetaminophen Hepatotoxicity Working Group. Recommendations for FDA interventions to decrease the occurrence of acetaminophen hepatotoxicity [report]. Feb 2008. https://www.litigationandtrial.com/files/2011/12/2009-4429b1-02-FDA.pdf (viewed Nov 2018).
- 20. Gunnell D, Hawton K, Murray V, et al. Use of paracetamol for suicide and non‐fatal poisoning in the UK and France: are restrictions on availability justified? J Epidemiol Community Health 1997; 51: 175–179.
- 21. Australian Department of Health, Therapeutic Goods Administration. Paracetamol: changes to pack size [media release]. 26 Aug 2013. https://www.tga.gov.au/media-release/paracetamol-changes-pack-size (viewed July 2019).
- 22. Chiew AL, Isbister GK, Page CB, et al. Modified release paracetamol overdose: a prospective observational study (ATOM‐3). Clin Toxicol 2018; 56: 810–819.
- 23. European Medicines Agency. Modified‐release paracetamol‐containing products to be suspended from EU market [EMA/118413/2018]. 19 Feb 2018. https://www.ema.europa.eu/medicines/human/referrals/paracetamol-modified-release (viewed Nov 2018).
- 24. Australian Department of Health, Therapeutic Goods Administration. Interim decision in relation to paracetamol (modified release) [Interim decisions and invitation for further comment on substances referred to the March 2019 ACMS/ACCS meetings]. 6 June 2019. https://www.tga.gov.au/book-page/15-interim-decision-relation-paracetamol-modified-release (viewed July 2019).
- 25. New South Wales Poisons Information Centre. 2013 annual report. https://www.poisonsinfo.nsw.gov.au/site/files/ul/data_text12/4918535-NSWPIC_Annual_Report_2013.pdf (viewed July 2019).





Abstract
Objectives: To assess the numbers of paracetamol overdose‐related hospital admissions and deaths in Australia since 2007–08, and the overdose size of intentional paracetamol overdoses since 2004.
Design, setting: Retrospective analysis of data on paracetamol‐related exposures, hospital admissions, and deaths from the Australian Institute of Health and Welfare National Hospital Morbidity Database (NHMD; 2007–08 to 2016–17), the New South Wales Poisons Information Centre (NSWPIC; 2004–2017), and the National Coronial Information System (NCIS; 2007–08 to 2016–17).
Participants: People who took overdoses of paracetamol in single ingredient preparations.
Main outcome measures: Annual numbers of reported paracetamol‐related poisonings, hospital admissions, and deaths; number of tablets taken in overdoses.
Results: The NHMD included 95 668 admissions with paracetamol poisoning diagnoses (2007–08 to 2016–17); the annual number of cases increased by 44.3% during the study period (3.8% per year; 95% CI, 3.2–4.6%). Toxic liver disease was documented for 1816 of these patients; the annual number increased by 108% during the study period (7.7% per year; 95% CI, 6.0–9.5%). The NSWPIC database included 22 997 reports of intentional overdose with paracetamol (2004–2017); the annual number increased by 77.0% during the study period (3.3% per year; 95% CI, 2.5–4.2%). The median number of tablets taken increased from 15 (IQR, 10–24) in 2004 to 20 (IQR, 10–35) in 2017. Modified release paracetamol ingestion report numbers increased 38% between 2004 and 2017 (95% CI, 30–47%). 126 in‐hospital deaths were recorded in the NHMD, and 205 deaths (in‐hospital and out of hospital) in the NCIS, with no temporal trends.
Conclusions: The frequency of paracetamol overdose‐related hospital admissions has increased in Australia since 2004, and the rise is associated with greater numbers of liver injury diagnoses. Overdose size and the proportion of overdoses involving modified release paracetamol have each also increased.