The known The incidence of cutaneous malignant melanoma has stabilised in recent years.
The new The incidence of invasive melanoma is declining among Victorians under 55 years of age, but is still increasing in those aged 55 or more, but at a slower rate than before the mid-1990s.
The implications Melanoma remains a significant health problem in Victoria. Prevention and early detection campaigns should take into account differences in presentation between men and women and in different age groups.
The most recent report on cancer in Australia by the Australian Institute of Health and Welfare recorded 12 744 new cases of invasive melanoma during 2013,1 making it the third most commonly diagnosed cancer in both men and women. Its incidence is greater in more northern parts of Australia; melanoma is the second most common cancer diagnosed in Queensland and New South Wales.2-4
Intermittent sun exposure and sunburn have long been recognised as major modifiable risk factors for melanoma.5,6 Accordingly, public health campaigns for the prevention of melanoma and of skin cancers in general have focused on sun protection.7 The stabilisation of the incidence of invasive melanoma in Australia has largely been attributed to these efforts.8,9
Monitoring the incidence of invasive melanoma remains important for policy makers, health care professionals, and the public. Understanding trends and characteristics in its incidence can guide health care professionals in identifying the patients at greatest risk, as well as informing the evaluation of public awareness campaigns and decisions about where future efforts should be directed. We therefore examined recent trends (1985–2015) in the incidence and characteristics of melanoma in Victoria.
Methods
Data source
We undertook a population-based, descriptive study of registry data. All Victorian Cancer Registry (VCR) records of melanoma diagnosed during 1985–2015 were reviewed. The VCR is managed by Cancer Council Victoria (CCV) on behalf of the Victorian Department of Health and Human Services. Hospitals and pathology laboratories in Victoria are legally required to report details of all diagnosed melanomas. The only inclusion criterion for our study was that patients were Victorian residents at the time of diagnosis. Our primary focus was invasive melanoma. Only the first instance of melanoma for a patient was included in the incidence analysis, consistent with standard international practice.10 Information recorded for each tumour included anatomic site (according to the International Classification of Diseases for Oncology, version 3 [ICD-O-3]) and Breslow thickness.
Statistical analysis
Annual estimates of the Victorian mid-year population by age were obtained from the Australian Bureau of Statistics.11 Age-standardised incidence and incidence by 5-year age band were calculated separately for men and women. Age-standardised incidence was estimated by weighting each 5-year age band incidence by the proportion of the 2015 Victorian population included in this age group. The entire Victorian population was included in the denominator for incidence calculations; we did not adjust for the increasing proportion of the population from ethnic groups at lower risk of melanoma.12
Changes in melanoma incidence were assessed in a joinpoint analysis.13 Joinpoint regression models are useful for assessing changes in incidence trends by identifying combinations of trends that provide a statistically significantly better fit to a data series than a single trend line. The logarithm of the age-standardised rates was assumed to have a linear trend between joinpoints, with errors following a Poisson distribution. Estimated annual percentage changes between joinpoints were determined from the fitted trends. Joinpoints were investigated by grid search and additional joinpoints were incrementally tested, with a minimum of four observations between joinpoints.
Cumulative lifetime risk was calculated as the sum of the age group-specific incidence rates, each multiplied by the duration of the age group.
The statistical independence of sex differences in the distribution of anatomic sites (ICD-O-3) and tumour thickness were analysed in χ2 tests, with Bonferroni correction for multiple comparisons. P < 0.05 was deemed statistically significant.
Calculations and statistical tests were performed with the MATLAB Statistics and Machine Learning Toolbox R2016b (MathWorks) and in Joinpoint 4.3.1.0 (United States National Cancer Institute).
Ethics approval
The investigators were granted approval from the CCV for access to and analysis of the data. The protocol for this study was approved by the Northern Sydney Local Health District Human Research Ethics Committee (reference, LNR/17/HAWKE/206).
Results
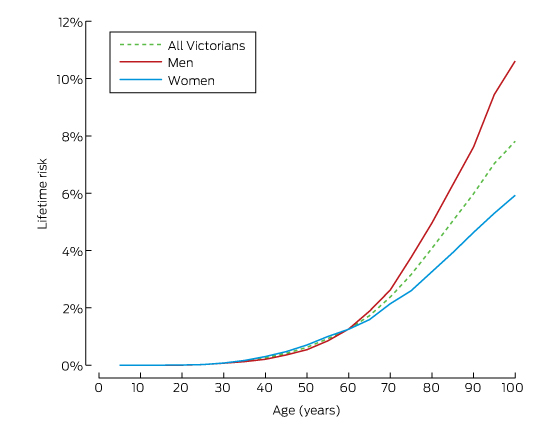
A total of 58 497 invasive melanoma tumours in 53 982 individuals were identified in the VCR; the registry also included 42 351 in situ melanoma tumours diagnosed during 1985–2015. In 2015, the incidence of invasive melanoma was 52.9 cases per 100 000 men and 39.2 cases per 100 000 women. Incidence increased with age for both sexes; in people under 50, the incidence was higher for women than men, but from age 50, the incidence was higher for men (online Appendix, table 1). These differences were reflected in the cumulative lifetime risks of melanoma for men and women in 2015 (Box 1). The cumulative lifetime risks of invasive melanoma at age 75 were 3.7% for men and 2.6% for women; at age 85, however, the cumulative risks were 6.3% and 3.9% respectively.
Joinpoint analysis of trends in age-standardised incidence identified a statistically significant slowing of the increase in incidence of invasive melanoma since the mid-1990s: from 1996 for men (P < 0.001) and from 1997 for women (P = 0.002). From 1985 to the joinpoint, the age-standardised incidence increased at an estimated annual rate of 5.3% (95% CI, 3.6–7.1%; P < 0.001) for men and at an estimated annual rate of 4.5% (95% CI, 2.9–6.1%; P < 0.001) for women. Since the 1996 joinpoint, the age-standardised incidence for men has increased at an estimated annual rate of 0.9% (95% CI, 0.3–1.5%; P = 0.006), but for women there has been no significant change since 1997 (estimated annual percentage change, –0.1%; 95% CI, –0.8% to 0.5%, P = 0.67) (Box 2).
When trends were analysed by 5-year age group, it was found that the incidence of invasive melanoma increased for both sexes during 1985–2015 for those aged 55 or more; in age groups under 55 years of age, the incidence declined or there was no significant change (data not shown). The age-standardised incidence for all people under 55 years of age increased by 2.7% per year (95% CI, 1.2–4.2%; P = 0.001) until 1996, after which it declined by 1.7% per year (95% CI, –2.5% to –0.9%; P < 0.001); for those aged 55 or more, the incidence rose by 5.8% per year (95% CI, 4.0–7.6%; P < 0.001) until 1996, and after this joinpoint by 1.6% per year (95% CI, 1.0–2.2%; P < 0.001). Similar trends applied to both sexes (Box 3).
The anatomic distribution of melanomas differed significantly between men and women (P < 0.001). The largest proportions of tumours in men were on the trunk (40%), in women on the upper (32%) and lower limbs (31%). Men had a greater proportion of tumours on the face (15% v 11%) and scalp and neck (10% v 4%) than women; the proportions of tumours on upper limb and shoulder (32% v 20%) and lower limb and hip (31% v 11%) were greater for women than for men (Box 4). The proportion of tumours on the face increased with age (in 2015: < 40 years, 8.3%; ≥ 80 years, 25% of all tumours, including those with unspecified anatomic location), while the proportion of tumours on the trunk was lower in those over 80 years of age than in younger age groups (in 2015: < 40 years, 31%; ≥ 80 years, 19% of all tumours) (online Appendix, table 2).
The distribution of tumour thickness classes was similar for both sexes in 2015 (P = 0.29; Box 4), and was fairly constant throughout the study period (data not shown). The proportion of thicker tumours (> 2 mm) was larger in older age groups of both sexes (online Appendix, table 3); the proportion was larger for men than for women (19% v 14%) (Box 4). Almost two-thirds (66%) of tumours more than 4 mm thick and less than one-third (32%) of those less than 1 mm thick were in people age 70 or more. The scalp and neck were the anatomic sites with the greatest proportion of thick tumours; the site with the greatest proportion of thin tumours was the trunk (online Appendix, table 4).
Finally, 1950 men (6.7%) and 1199 women (4.8%) with a diagnosis of malignant melanoma in our sample were subsequently diagnosed with a further malignant melanoma.
Discussion
The incidence and cumulative lifetime risk of invasive melanoma in Victoria was higher in men than in women; in 2015, one in 16 men developed invasive melanoma by the age of 85, compared with one in 26 women. The difference is largely attributable to the higher incidence in older men. Since the mid-1990s, the increase in the incidence of invasive melanoma has slowed in both sexes; in women, the rate has plateaued. The incidence remains higher in more northerly states, but the trends are similar to those in other Australian states and for Australia overall.3,4,8,9 However, our findings are not congruent with a recent report than the incidence of melanoma is declining in Australia.9 The inconsistency may reflect differences in trends for different states; for example, there is greater latitude in Queensland for improvement because of the relatively higher incidence.2,14 Further, our study covered 4 more years than the earlier report;9 together with differences in the base populations used for age standardisation, this may also have contributed to our divergent findings.
The incidence of melanoma is higher in Australia and New Zealand than elsewhere in the world,8,9 but is rising in the United States and several European countries, including Norway and Sweden, where the overall incidence is about 30 cases per 100 000 population.8,9 In Europe, the incidence in several countries has been rising by about 5% each year (eg, Norway and Sweden, 2004–2011; United Kingdom, 1991–2011).9 However, the incidence of melanoma in New Zealand and the United States appears to have stabilised,8,9 similar to our findings for Victoria.
A promising finding was the declining incidence in people under 55 years of age of both sexes, with annual decreases since the mid-1990s of 1.4% for men and 1.9% for women. Similar age-related differences have been reported in the US.15 More substantial decreases in younger age groups have been reported in Queensland: annual declines of 3.8% (men) and 3.4% (women) for people under 40, and of 2.6% (men) and 1.7% (women) for people aged 40–59 years, with the incidence stable for those aged 60 years or more.14 In Victoria, the overall incidence of melanoma is elevated by the continued increases in incidence among older people, but it may stabilise as the rate of increase in older age groups decreases as currently younger people reach these age groups.
These results attest to the effectiveness of skin cancer prevention campaigns since the early 1980s, such as the Slip! Slop! Slap! and SunSmart campaigns.7 Given the long latency between excessive sun exposure and the clinical presentation of melanoma, the first observable changes attributable to primary prevention campaigns would have reasonably been expected in the mid-1990s.
Skin cancer prevention campaigns also promote early detection, and the slowing increase in the incidence of invasive melanoma may also indicate that more melanomas are being diagnosed while still in situ. Studies in Victoria, other Australian states and overseas have also found that the incidence of melanoma in situ is increasing at a faster rate than that of invasive melanoma;16-20 this phenomenon could also be investigated in VCR data. However, our findings provide little support for the view that an increasing proportion of invasive melanoma tumours are thin tumours.
Melanoma thickness is a key prognostic marker,21,22 and we found little change in the distribution of the thickness of invasive tumours over time. The higher proportions of face, scalp and neck tumours in older age groups, particularly in older men, could be related to occupational and lifelong sun exposure rather than intermittent extreme exposure,23,24 and a greater proportion of tumours at these anatomic sites were thick tumours. The persistence of thick tumours might be partially explained by the frequency of nodular melanomas, which grow rapidly and are difficult to detect early.25
The difference between men and women in the anatomic distribution of melanoma tumours was marked. The higher proportions of tumours on the face, scalp and neck in men and of tumours on the upper and lower limbs in women may both be explained by current clothing and grooming trends, with men generally having shorter hair and women more likely to routinely expose more of their limbs more frequently.
The risk of subsequent melanoma after an initial melanoma diagnosis is widely recognised. Standardised incidence ratios (SIRs) in Australia for the excess risk following a diagnosis of cancer are generally lower than for other countries because of the high background incidence of melanoma.26,27 The VCR has previously reported SIRs for men of 7.9 (under 65 years) and 6.6 (65 or over) and for women of 7.3 (under 65 years) and 7.2 (65 or over).26 We could not calculate accurate SIRs because the population at risk could not be defined without data on deaths.
There is an opportunity to both improve patient health and to achieve economic savings. The cost-effectiveness of skin cancer prevention campaigns has been demonstrated.28,29 Similarly, identifying and monitoring patients at high risk of melanoma has been shown to provide better patient outcomes and to be cost-effective.30 To complement the skin cancer prevention messages that are largely directed to the young, public awareness campaigns could specifically target older people and men to improve earlier detection by encouraging self-screening and having suspicious lesions and moles investigated. Promoting early detection in these groups, in which the incidence of invasive melanoma is highest, could reduce the prevalence of thick tumours, which are associated with poor prognoses and high economic costs.21,30
One limitation of our study was that our analysis was restricted to Victoria. Further, investigating trends in melanoma subtype would also be a useful extension of our analysis.
Our finding that growth in the incidence of invasive melanoma in Victorians under 55 has slowed since the mid-1990s is encouraging. However, complacency is not appropriate. Melanoma remains a significant health problem in Victoria, and its incidence is still increasing in older people. Less encouraging is that the proportion of thicker tumours remains steady and therefore, despite recent progress in systemic therapies, continues to be a problem. The higher rates of thicker tumours in older people show the importance of early detection in these patients, and this should be the focus of public awareness campaigns.
Box 2 – Age-standardised incidence of invasive melanoma, by year and sex (A, males; B, females), with joinpoint analysis-fitted trends
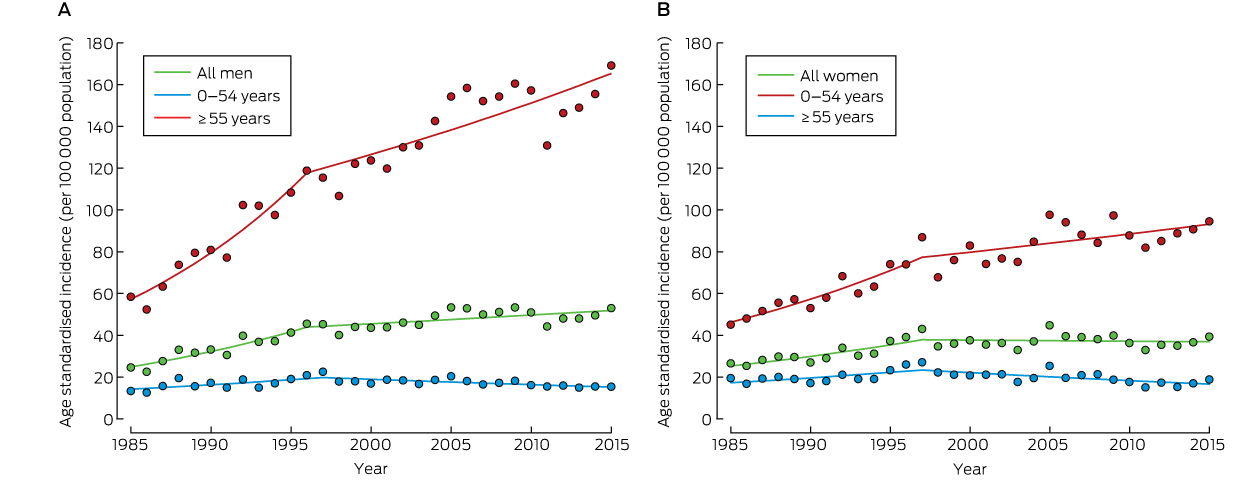
Box 3 – Age-standardised incidence of invasive melanoma, in Victoria, 2015, and estimated annual percentage changes in incidence, by sex and joinpoint segment*
|
|
Age-standardised incidence (2015), per 100 000 population |
Annual percentage change (95% confidence interval) |
|||||||||||||
|
1985 to joinpoint |
P |
Joinpoint to 2015 |
P |
||||||||||||
|
|
|||||||||||||||
|
Men |
52.9 |
5.3% (3.6–7.1%) |
< 0.001 |
0.9% (0.3–1.5%) |
0.006 |
||||||||||
|
0–54 years |
15.3 |
2.8% (1.2–4.4%) |
0.002 |
–1.4% (–2.3 to –0.6%) |
0.002 |
||||||||||
|
≥ 55 years |
169.0 |
6.8% (4.7–8.9%) |
< 0.001 |
1.8% (1.1–2.5%) |
< 0.001 |
||||||||||
|
Women |
39.2 |
3.4% (2.0–4.9%) |
< 0.001 |
–0.1% (–0.8 to 0.5%) |
0.67 |
||||||||||
|
0–54 years |
18.8 |
2.6% (0.8–4.3%) |
0.006 |
–1.9% (–2.8 to –0.9%) |
< 0.001 |
||||||||||
|
≥ 55 years |
94.5 |
4.4% (2.9–5.9%) |
< 0.001 |
1.0% (0.4–1.7%) |
0.003 |
||||||||||
|
Total |
46.0 |
4.5% (2.9–6.1%) |
< 0.001 |
0.5% (–0.1 to 1.1%) |
0.07 |
||||||||||
|
0–54 years |
17.1 |
2.7% (1.2–4.2%) |
0.001 |
–1.7% (–2.5 to –0.9%) |
< 0.001 |
||||||||||
|
≥ 55 years |
129.7 |
5.8% (4.0–7.6%) |
< 0.001 |
1.6% (1.0–2.2%) |
< 0.001 |
||||||||||
|
|
|||||||||||||||
|
* Joinpoint for men and total: 1996; for women, 1997. |
|||||||||||||||
Box 4 – Anatomic location and thickness of invasive melanoma tumours, Victoria, 2015
|
|
Number of tumours (proportion of total number) |
||||||||||||||
|
Men |
Women |
Total |
|||||||||||||
|
|
|||||||||||||||
|
Total number of tumours |
1554 |
1178 |
2732 |
||||||||||||
|
Anatomic site |
|
|
|
||||||||||||
|
Face* |
237 (15.3%) |
126 (10.7%) |
363 (13.3%) |
||||||||||||
|
Scalp/neck |
150 (9.7%) |
49 (4.2%) |
199 (7.3%) |
||||||||||||
|
Trunk |
616 (39.6%) |
213 (18.1%) |
829 (30.3%) |
||||||||||||
|
Upper limb/shoulder |
311 (20.0%) |
379 (32.2%) |
690 (25.3%) |
||||||||||||
|
Lower limb/hip |
175 (11.3%) |
368 (31.2%) |
543 (19.9%) |
||||||||||||
|
Unspecified or overlap |
65 (4.2%) |
43 (3.7%) |
108 (4.0%) |
||||||||||||
|
Breslow thickness |
|
|
|
||||||||||||
|
≤ 1 mm |
921 (59.3%) |
769 (65.3%) |
1690 (61.9%) |
||||||||||||
|
> 1 mm to 2 mm |
214 (13.8%) |
158 (13.4%) |
372 (13.6%) |
||||||||||||
|
> 2 mm to 4 mm |
182 (11.7%) |
96 (8.1%) |
278 (10.2%) |
||||||||||||
|
> 4 mm |
111 (7.1%) |
69 (5.9%) |
180 (6.6%) |
||||||||||||
|
Unspecified |
126 (8.1%) |
86 (7.3%) |
212 (7.8%) |
||||||||||||
|
|
|||||||||||||||
|
* Includes lip, eyelid, and ear. |
|||||||||||||||
Received 28 July 2017, accepted 19 January 2018
- David J Curchin1
- Victoria R Harris2
- Christopher J McCormack3
- Saxon D Smith1,2
- 1 Northern Clinical School, University of Sydney, Sydney, NSW
- 2 Royal North Shore Hospital, Sydney, NSW
- 3 Peter MacCallum Cancer Centre, Melbourne, VIC
No relevant disclosures.
- 1. Australian Institute of Health and Welfare. Cancer in Australia 2017 (AIHW Cat. No. CAN 100; Cancer Series No. 101). Canberra: AIHW, 2017. https://www.aihw.gov.au/reports/cancer/cancer-in-australia-2017 (viewed Feb 2017).
- 2. Baade P, Meng X, Youlden D, et al. Time trends and latitudinal differences in melanoma thickness distribution in Australia, 1990–2006. Int J Cancer 2012; 130: 170-178.
- 3. Cancer Institute NSW. Cancer in NSW 2017. May 2017. https://www.cancerinstitute.org.au/cancer-data-pages (viewed May 2017).
- 4. Cancer Council Queensland. Queensland cancer statistics online (QCSOL). 2017. https://cancerqld.org.au/research/queensland-cancer-statistics/queensland-cancer-statistics-online-qcsol/ (viewed Apr 2017).
- 5. Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control 2001; 12: 69-82.
- 6. Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer 2005; 41: 45-60.
- 7. Montague M, Borland R, Sinclair C. Slip! Slop! Slap! and SunSmart, 1980–2000: skin cancer control and 20 years of population-based campaigning. Health Educ Behav 2001; 28: 290-305.
- 8. Erdmann F, Lortet-Tieulent J, Schuz J, et al. International trends in the incidence of malignant melanoma 1953–2008 — are recent generations at higher or lower risk? Int J Cancer 2013; 132: 385-400.
- 9. Whiteman DC, Green AC, Olsen CM. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol 2016; 136: 1161-1171.
- 10. Boyle P, Parkin DM. Statistical methods for registries. In: Jensen OM, Parkin DM, MacLennan R, et al (editors), Cancer registration: principles and methods (IARC Scientific Publications No. 95). Lyon: International Agency for Research on Cancer, 1991; pp. 126-158. http://www.iarc.fr/en/publications/pdfs-online/epi/sp95/sp95-chap11.pdf (viewed May 2017).
- 11. Australian Bureau of Statistics. 3101.0. Australian demographic statistics, June 2016 (table 52). Dec 2016. http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/3101.0Jun%202016?OpenDocument (viewed May 2017).
- 12. Baade PD, Youlden DR, Youl P, et al. Assessment of the effect of migration on melanoma incidence trends in Australia between 1982 and 2010 among people under 30. Acta Derm Venereol 2015; 95: 118-120.
- 13. Kim H, Fay M, Feuer E, Midthune D. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000; 19: 335-351 (correction: 2001; 20: 655).
- 14. Youl PH, Youlden DR, Baade PD. Changes in the site distribution of common melanoma subtypes in Queensland, Australia over time: implications for public health campaigns. Br J Dermatol 2013; 168: 136-144.
- 15. Holman DM, Freeman MB, Shoemaker ML. Trends in melanoma incidence among non-Hispanic whites in the United States, 2005 to 2014. JAMA Dermatol 2018; doi:10.1001/jamadermatol.2017.5541 [Epub ahead of print].
- 16. Coory M, Baade P, Aitken J, et al. Trends for in situ and invasive melanoma in Queensland, Australia, 1982–2002. Cancer Causes Control 2006; 17: 21-27.
- 17. Wei EX, Qureshi AA, Han J, et al. Trends in the diagnosis and clinical features of melanoma in situ (MIS) in US men and women: a prospective, observational study. J Am Acad Dermatol 2016; 75: 698-705.
- 18. Toender A, Kjaer SK, Jensen A. Increased incidence of melanoma in situ in Denmark from 1997 to 2011: results from a nationwide population-based study. Melanoma Res 2014; 24: 488-495.
- 19. Smithson SL, Pan Y, Mar V. Differing trends in thickness and survival between nodular and non-nodular primary cutaneous melanoma in Victoria, Australia. Med J Aust 2015; 203: 20. <MJA full text>
- 20. Meani RE, Pan Y, McLean C, et al. The Victorian Melanoma Service: a 20-year review of an Australian multidisciplinary cancer service. Australas J Dermatol 2016; 57: 235-237.
- 21. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27: 6199-6206.
- 22. Payette MJ, Katz M, Grant-Kels JM. Melanoma prognostic factors found in the dermatopathology report. Clin Dermatol 2009; 27: 53-74.
- 23. Whiteman DC, Stickley M, Watt P, et al. Anatomic site, sun exposure, and risk of cutaneous melanoma. J Clin Oncol 2006; 249: 3172-3177.
- 24. Anderson WF, Pfeiffer RM, Tucker MA, Rosenberg PS. Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer 2009; 115: 4176-4185.
- 25. Mar V, Roberts H, Wolfe R, et al. Nodular melanoma: a distinct clinical entity and the largest contributor to melanoma deaths in Victoria, Australia. J Am Acad Dermatol 2013; 68: 568-575.
- 26. Karahalios E, English D, Thursfield V, et al. Second primary cancers in Victoria [Internet]. Melbourne: Cancer Council Victoria, 2009. http://www.cancervic.org.au/downloads/cec/Second-Primary-Cancers.pdf (viewed May 2017).
- 27. van der Leest RJ, Flohil SC, Arends LR, et al. Risk of subsequent cutaneous malignancy in patients with prior melanoma: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2015; 29: 1053-1062.
- 28. Hirst NG, Gordon LG, Scuffham PA, Green AC. Lifetime cost-effectiveness of skin cancer prevention through promotion of daily sunscreen use. Value Health 2012; 15: 261-268.
- 29. Doran CM, Ling R, Byrnes J, et al. Benefit cost analysis of three skin cancer public education mass-media campaigns implemented in New South Wales, Australia. PLoS One 2016; 11: e0147665.
- 30. Watts CG, Cust AE, Menzies SW, et al. Cost-effectiveness of skin surveillance through a specialized clinic for patients at high risk of melanoma. J Clin Oncol 2017; 35: 63-71.





Abstract
Objectives: To estimate the incidence of cutaneous malignant melanoma in Victoria; to examine trends in its incidence over the past 30 years. Secondary objectives were to examine the anatomic location and thickness of invasive melanoma tumours during the same period.
Design: Population-based, descriptive analysis of Victorian Cancer Registry data.
Participants: Victorian residents diagnosed with melanoma, 1985–2015.
Main outcome measures: Age-standardised incidence of invasive melanoma; estimated annual percentage changes in incidence.
Results: In 2015, the incidence of invasive melanoma in Victoria was 52.9 cases per 100 000 men and 39.2 cases per 100 000 women. Since the mid-1990s, the incidence for men increased annually by 0.9% (95% CI, 0.3–1.5%), but for women there was no significant change (estimated annual percentage change, –0.1%; 95% CI, –0.8% to 0.5%). The incidence of invasive melanoma has been declining in age groups under 55 years of age since 1996 (overall annual change, –1.7%; 95% CI, –2.5% to –0.9%), but is still increasing in those over 55 (overall annual change, 1.6%; 95% CI, 1.0–2.2%). The most frequent site of tumours in men was the trunk (40%), on women the upper (32%) and lower limbs (31%).
Conclusions: Melanoma remains a significant health problem, warranting continued prevention efforts. Awareness of differences in presentation by men and women and in different age groups would facilitate improved screening and risk identification.