The known A polyvalent serogroup B meningococcus (MenB) vaccine was licensed in Australia in 2014, but data on the age-specific incidence of MenB disease that could guide individual risk–benefit assessments are limited.
The new During 2006–2015, the incidence of MenB disease decreased markedly, but constituted about 81% of all invasive meningococcal disease cases with known serogroup. Its incidence was highest in children under 12 months of age (11.1 cases per 100 000); it was almost four times as high among Indigenous as among non-Indigenous Australians, and was particularly high among Indigenous children.
The implications Priority at risk age/population groups for MenB vaccination include all children between 2 months and 5 years of age, Indigenous children under 10 years of age, and all adolescents aged 15–19 years.
Neisseria meningitidis is a leading cause of life-threatening septicaemia and meningitis in children worldwide. The varying prevalence of serogroups A, B, C, W and Y by region that cause acute, sporadic cases or epidemic events contributes to its highly diverse global epidemiology.1,2 The incidence of invasive meningococcal disease (IMD) infections is typically low in high income countries such as Australia, where sporadic group B infections predominate.2,3
Even with good access to medical care, IMD can rapidly progress to death.4 About one in ten survivors of meningococcal B (MenB) disease develop major disabling sequelae, and more than one-third may have one or more physical, cognitive, psychological, memory, or executive function deficits,5 incurring both personal and societal costs.6 The likelihood of severe outcomes and the challenges of early diagnosis and treatment, combined with public fears about the disease, make prevention of IMD highly desirable.
Since the meningococcal C conjugate vaccines (MenCCV) were added to the National Immunisation Program in 2003, the incidence of group C meningococcal disease in Australia has remained very low among both vaccinated and unvaccinated people. This finding of indirect protection (herd immunity) is consistent with observations of reduced carriage prevalence among vaccinated adolescents in the United Kingdom.7,8 The recent development of two MenB vaccines that afford protection against multiple strains provides new opportunities for preventing group B disease. In August 2013, a multicomponent recombinant vaccine designed to afford protection against a broad spectrum of MenB strains (Bexsero, manufactured by Novartis/GSK) was registered in Australia for vaccinating people at least 2 months old. Since August 2014, this vaccine has been available in Australia by private prescription; the Australian Technical Advisory Group on Immunisation (ATAGI) has subsequently recommended its targeted use in age groups and other populations at increased risk of invasive disease.9 The Pharmaceutical Benefits Advisory Committee rejected successive applications by industry to have the vaccine included in the National Immunisation Program.10,11
A recent Australian study reported that recommendations by the family doctor had the strongest influence on parents’ willingness to have their child receive a MenB vaccine.12 There is, however, limited published information on trends in the age-specific risk of invasive group B disease in Australia, especially among those under 2 years of age. We therefore examined the age-specific incidence of invasive MenB disease in Australia during 1999–2015, with the aim of identifying age and population groups at risk of invasive MenB disease, to inform national policy deliberations and individual risk–benefit assessments of MenB vaccination in the absence of a national program.
Methods
In our observational study, we analysed de-identified notification data from the Australian National Notifiable Diseases Surveillance System (NNDSS) in February 2017. Our study included notifications of IMD from all Australian states and territories with a recorded diagnosis date during 1999–2015.
Case definition
Since late 2004, a set of national case definitions for notification to the NNDSS of both probable and confirmed cases of IMD has been adopted by all Australian jurisdictions.13 We included both probable and confirmed cases in our overall analysis; analyses by serogroup were limited to confirmed cases only. We detected internal inconsistencies of confirmation status in the dataset, necessitating reclassification of some cases according to laboratory confirmation or typing data, applying a set of rules and assumptions approved by ATAGI (online Appendix, table 1).
Statistical analysis
Infants under 2 years of age with recorded dates of birth and dates of onset of IMD (or, when not available, specimen collection date) were categorised into one of five age groups (0–2, 3–6, 7–8, 9–11, 12–23 months). The recommended schedule for infants of the licensed MenB vaccine includes doses at ages 2, 4, 6 and 12 months.
Given the high ascertainment rate of IMD cases by the NNDSS14, we assumed that notifications accurately reflected the incidence of disease. Denominators for calculating incidence rates were based on published Australian 1999–2015 mid-year resident population projections, including estimates of total population by age in single years and state or territory15 and of the Aboriginal and Torres Strait Islander (Indigenous) population.16 We imputed the non-Indigenous population as the difference between total and Indigenous population estimates. To explore any difference in distribution of age at disease onset by serogroup, while also allowing for adequate case counts, average annual incidence rates per 100 000 person-years were calculated for MenB notifications during the most recent 10-year period (2006–2015). These rates were compared with MenC notifications during 1999–2002, the period when the proportion of confirmed cases with unknown or undetermined serogroup declined to under 30%, but before the introduction of the MenCCV.
Statistical analyses were performed in SAS Enterprise Guide 6.1 (SAS Institute). A Poisson distribution was assumed for estimating 95% confidence intervals (CIs) for incidence rates. Case fatality rates by serogroup were compared in Fisher exact tests. Incidence rate ratios (with 95% CIs) were calculated for assessing the significance of differences between age-specific rates by Indigenous status. P < 0.05 (two-sided) was deemed statistically significant.
Ethics approval
Access to the de-identified NNDSS data extract for this epidemiological analysis was approved by the Communicable Diseases Network Australia (http://www.health.gov.au/cdna). It was deemed that specific ethics approval for the study was not required.
Results
During 1999–2015, 6306 notifications of IMD were recorded by the NNDSS, of which 5968 (95%) met the case definition of confirmed cases, including 94 re-classified as described above.
Longitudinal trends
Following a peak in 2001 (3.54 per 100 000 population), the overall incidence of IMD progressively declined to 0.64 cases per 100 000 in 2013, then increased to 0.77 per 100 000 in 2015 (Box 1). The proportion of confirmed IMD cases for which the causative serogroup was determined increased from 77% in 1999 to 96% in 2015. Serogroup B was the most common serogroup overall and in each individual year during the 17-year period, including 1999–2002 (ie, prior to implementation of the national MenCCV program). After untyped cases were excluded, MenB constituted 83% (range, 76–88%) of IMD cases during 2006–2014, but this declined to 65% in 2015 as a result of increases in contributions by serogroups W and Y, resulting in an overall contribution of 81% of IMD cases during 2006–2015. While the effect of the MenCCV program on the incidence of group C disease was apparent (99% decline in case incidence since 2002), the incidence of MenB disease also declined, from 1.52 cases per 100 000 in 2001 to 0.47 per 100 000 population in 2015 (69% decrease). Very low rates for other serogroups were observed during 1999–2014, before the clear emergence of MenW in Australia in 2015 (when the rate increased to 0.14 per 100 000), with an average of ten cases each (0.05 per 100 000) for serogroups W and Y each year. Few cases were caused by other serogroups during the study period (A, five cases; X, two cases).
Age distribution of the two predominant serogroups (B and C)
MenB and MenC disease each had asymmetric bimodal age incidence distributions (Box 2), but with different peak ages. During 2006–2015, a mean of 174 confirmed cases of MenB disease were reported annually, including a mean 32 cases in infants under 12 months of age, 32 in children aged 2–4 years, and 35 in patients aged 15–19 years (Box 3). The peak incidence was in infants under 12 months of age (11.1 cases per 100 000), particularly among those aged 3–6 months (15.1 per 100 000). Smaller peak rates were noted for children aged 1–4 years (2.82 per 100 000) and adolescents aged 15–19 years (2.40 per 100 000). Of the 473 infants under 2 years with MenB disease during 2006–2015, 43% were under 7 months old and 69% under 12 months old (data not shown).
In contrast, the peak incidence of MenC disease during 1999–2002, prior to the MenCCV program, was at 18 years (Box 2). In infants under 12 months of age, the incidence (2.60 per 100 000) was one-quarter that of MenB, but was slightly higher among young people aged 15–19 years (3.32 per 100 000).
The observed bimodal age distribution for MenB also applied to each calendar year included in the study. The age distribution of MenC disease, however, was less uniform from 2006 onwards, with sporadic cases across the age spectrum (data not shown).
Deaths (case fatality rates)
The completeness of reporting of case outcomes for all IMD notifications improved from 26% in 1999 to 79% in 2015. Assuming that all patients with unknown outcomes survived, the estimated all-age case fatality rate for MenB infections was 4%, and 8% for MenC infections (P < 0.01).
Serogroup-specific death rates by age differed markedly (Box 4). Although most IMD deaths in children under 5 (83%) were linked to serogroup B infections (data not shown), the case fatality rates for MenB and MenC were similar in this age group (5%). In contrast, the case fatality rates were significantly higher for MenC than for MenB disease among adolescents aged 15–24 years (P < 0.01) and adults over 25 (P < 0.01).
Indigenous Australians
Indigenous status of the patient was recorded for 97% of MenB notifications during 2006–2015. The all-age incidence of MenB disease was significantly higher among Indigenous than among non-Indigenous Australians (incidence rate ratio, 3.8; 95% CI, 3.3–4.5). This difference was evident across most groups under 25 years of age, and was most pronounced in children under 10 (Box 5). Overall MenB case fatality rates were similar for Indigenous (3%) and non-Indigenous Australians (4%). In contrast to the progressive decline in MenB incidence in non-Indigenous populations, the all-age incidence of MenB in Indigenous Australians has been more variable, fluctuating between 1.65 and 4.51 cases per 100 000 during 2006–2015 (data not shown).
Geographic distribution
Most MenB notifications during 1999–2015 were from New South Wales (1089 cases, 30%), Queensland (809 cases, 22%) and Victoria (752 cases, 20%). Historically, the incidence of MenB disease has been greatest in the Northern Territory, but it has also declined here, from a peak of 4.6 cases per 100 000 in 2003 to 0.4 per 100 000 in 2015 (online Appendix, table 4). The incidence in South Australia remained high throughout the study period, with the highest value in 2015 (1.65 cases per 100 000).
Discussion
IMD has been increasingly uncommon in Australia since 2003, the year the national MenC vaccination campaign commenced. Since 2006, serogroup B disease has accounted for most confirmed IMD cases (83%). The highest incidence of MenB disease was for infants under 12 months of age (43% before the earliest receipt of the third MenB vaccine dose at 6 months), followed by young children (1–4 years) and older adolescents (15–19 years). The incidence of MenB disease was significantly higher among Indigenous than other Australians, particularly among children under 10 years of age.
Context for the current epidemiology of MenB disease is provided by comparing it with that of MenC before the MenCCV was available. First, the peak incidence of MenC disease was in late adolescence, rather than in infancy and early childhood; second, case fatality rates for adolescent patients were higher for MenC disease; third, there was no disparity between Indigenous Australians and others in the incidence of MenC.17 This epidemiological profile led to selecting the schedule of a single dose of MenCCV at age 12 months or later (initial or catch-up doses); together with an extensive catch-up campaign for children and adolescents, this markedly reduced MenC incidence to its current low levels through a combination of direct and indirect (herd immunity) effects.7,8,18
In contrast, our epidemiological data indicate that the morbidity and mortality of MenB disease are greatest during infancy, particularly the first 8 months of life. As the impact of the multicomponent MenB vaccine on carriage and, consequently, the potential indirect protective effects achievable by vaccinating older people remain speculative,19 increasing direct protection by vaccinating younger infants is the strategy most likely to yield the greatest benefit, despite the scheduling challenges and concerns about vaccine reactions, especially fever.20
Since 1999, the overall incidence of MenB disease in Australia has declined, to a historic low of fewer than 0.5 cases per 100 000 population in 2015. Similar declines without a specific MenB vaccination program have been reported in other industrialised countries. However, the incidence in Australia remains notably higher than the sustained low rates in Canada (0.3 per 100 000 during 2006–2011)6 and the United States (fewer than 0.1 cases per 100 000 during 2002–2011),21 including among infants.22 Somewhat higher (but gradually declining) rates of MenB disease have been reported in England among infants under 12 months of age (12–19 per 100 000),23 which contributed to the decision to include the MenB vaccine in the UK immunisation program, with cost-effectiveness improved by a reduced dose schedule (two doses during infancy, one booster at 12 months).24 The first assessment of the impact on this schedule found that the number of cases among vaccine-eligible infants had declined by 50% during the first 10 months of the program;25 if sustained, this would have implications for cost-effectiveness as well as for disease trends.
The epidemiology of MenB disease is changing in Australia; its overall incidence has remained stable, but increased numbers of infections by serogroups W and Y since 20133,26 means that it accounted for 65% of IMD cases with a known serogroup during 2015, down from 83% during 2006–2014. There is also some regional variation; while incidence has declined in the three most populous states, the rate in the Northern Territory has been more variable, and has increased in South Australia, where it prompted the initiation of a large comparative study of MenB vaccination of adolescents in 2017.27
Our study has several limitations. Routine notification data have been influenced by changes in case definition, regional data management practices, and data completeness. The strength of laboratory evidence has also varied. Australian public health authorities regard notification data for the most recent years assessed, and following wide availability of nucleic acid detection assays from 2002, as robust — recent studies linking notification data with birth, hospital and death records14 have found only a low level of missed cases — allowing an accurate estimate of disease incidence.
As a MenB vaccine is available in Australia, our epidemiological findings will assist identify key target groups for vaccination, and will be critical when evaluating the limitations, cost-effectiveness, and potential configuration of a national MenB vaccination program. Our findings should nevertheless be considered together with information on the vaccine-preventable fraction (ie, the proportion of incidence averted by vaccination), schedule requirements, and vaccine efficacy. Indeed, the decision of the Pharmaceutical Benefits Advisory Committee to not include MenB vaccine in the National Immunisation Program highlights the uncertainty about the protection afforded at the time of its review, the extent and duration of effect, variability in MenB strain coverage, and the potential for herd immunity.11
Prior to the first large scale application of the multicomponent MenB vaccine in the UK,25 proprietary laboratory assays predicted protection against 76% of MenB strains that cause disease in Australia.28 Uncertainty remains as to whether this will prove to be optimistic, given marked changes in serogroup and subtype prevalence,3,29,30 or whether the vaccine will provide protection beyond the B serotype, potentially including MenW, which could mean that its overall protective effect has been underestimated. Vaccine safety considerations are also paramount in individual risk–benefit assessments. The high probability of systemic reactions, such as high fever in children under 2 years of age, prompted ATAGI to recommend prophylactic use of paracetamol when administering the vaccine in this age group.9
In the absence of funding by the National Immunisation Program, Australia is at risk of falling victim to the inverse care law,31 in that access to and availability of the MenB vaccine may be lowest among the highest risk population groups we identified in this study. However, clinical advice on MenB vaccination at the level of the person is still needed, and our data will assist doctors, families and individuals balance the expected benefit afforded by vaccination against the likelihood of disease, the risks of potential reactions, and the costs of vaccination.
Further details on ATAGI are available from: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/atagi.
Box 1 – Secular trends in the incidence of notifications of invasive meningococcal disease, Australia, 1999–2015, by serogroup*
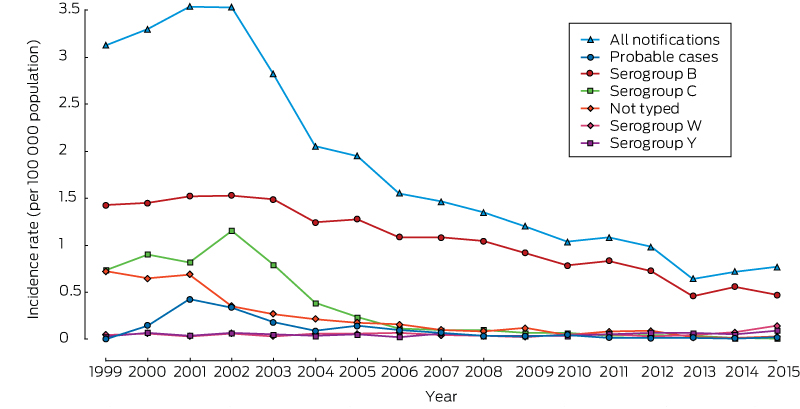
* Data for serogroups A (five cases) and X (two) are not shown. The complete data table for all serogroups is included in the online Appendix as table 2.
Box 2 – Age distribution of confirmed cases of invasive meningococcal serogroups B (2006–2015) and C disease (1999–2002), Australia*
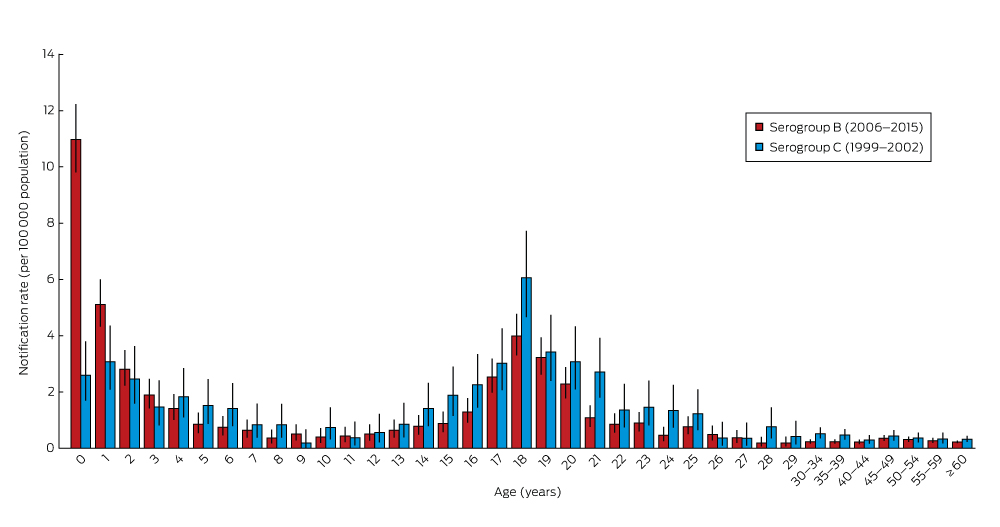
* Error bars show 95% confidence interval for incidence rate estimates. The complete data table is included in the online Appendix as table 3.
Box 3 – Age distribution of confirmed cases of invasive meningococcal serogroup B disease, Australia, 2006–2015
|
Age group |
Person-years, 2006–2015 |
Number of cases (proportion) |
Annual number of cases (mean) |
Incidence: cases per 100 000 person-years (95% CI) |
|||||||||||
|
|
|||||||||||||||
|
< 12 months |
2 952 909 |
324 (19%) |
32 |
11.1 (9.81–12.2) |
|||||||||||
|
0–2 months |
738 227 |
56 [17%*] |
6 |
7.59 (5.73–9.85) |
|||||||||||
|
3–6 months |
984 303 |
149 [46%*] |
15 |
15.1 (12.8–17.8) |
|||||||||||
|
7–8 months |
492 152 |
59 [18%*] |
6 |
12.0 (9.13–15.5) |
|||||||||||
|
9–11 months |
738 227 |
60 [19%*] |
6 |
8.13 (6.20–10.5) |
|||||||||||
|
1–4 years |
11 483 418 |
324 (19%) |
32 |
2.82 (2.52–3.15) |
|||||||||||
|
5–14 years |
27 883 626 |
164 (9%) |
16 |
0.59 (0.50–0.69) |
|||||||||||
|
15–19 years |
14 515 186 |
349 (20%) |
35 |
2.40 (2.16–2.67) |
|||||||||||
|
20–24 years |
15 831 572 |
175 (10%) |
18 |
1.11 (0.95–1.28) |
|||||||||||
|
≥ 25 years |
149 020 142 |
405 (23%) |
41 |
0.27 (0.25–0.30) |
|||||||||||
|
All ages |
221 686 853 |
1741 (100%) |
174 |
0.79 (0.75–0.82) |
|||||||||||
|
|
|||||||||||||||
|
* Proportion of cases in infants under 12 months of age. |
|||||||||||||||
Box 4 – Case fatality rates (CFRs) for meningococcal serogroups B and C disease, Australia, 1999–2015, by age group
|
Age group |
Serogroup B |
Serogroup C |
|||||||||||||
|
Cases |
Deaths |
CFR |
Cases |
Deaths |
CFR |
||||||||||
|
|
|||||||||||||||
|
< 5 years |
1450 |
72 |
5% |
164 |
9 |
5% |
|||||||||
|
5–9 years |
237 |
4 |
2% |
73 |
6 |
8% |
|||||||||
|
10–14 years |
183 |
1 |
1% |
65 |
1 |
2% |
|||||||||
|
15–24 years |
1013 |
25 |
2% |
416 |
27 |
6% |
|||||||||
|
≥ 25 years |
792 |
51 |
6% |
381 |
44 |
12% |
|||||||||
|
All ages |
3675 |
153 |
4% |
1099 |
87 |
8% |
|||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Box 5 – Meningococcal serogroup B disease notifications, Australia, 2006–2015, by Indigenous status and age group
|
Age group |
Indigenous |
Non-Indigenous, or status unknown |
Incidence rate ratio (95% CI) |
||||||||||||
|
Person-years, |
Number of cases (IR) |
Person-years, |
Number of cases (IR) |
||||||||||||
|
|
|||||||||||||||
|
< 12 months |
169 378 |
55 (32.5) |
2 783 531 |
269 (9.66) |
3.4 (2.5–4.5) |
||||||||||
|
1–4 years |
658 142 |
61 (9.27) |
10 825 276 |
263 (2.43) |
3.8 (2.9–5.0) |
||||||||||
|
5–9 years |
799 097 |
24 (3.00) |
13 158 233 |
63 (0.48) |
6.3 (3.9–10) |
||||||||||
|
10–14 years |
771 071 |
10 (1.30) |
13 155 225 |
67 (0.51) |
2.5 (1.3–4.9) |
||||||||||
|
15–24 years |
1 313 584 |
18 (1.37) |
29 033 174 |
506 (1.74) |
0.8 (0.5–1.3) |
||||||||||
|
≥ 25 years |
2 929 550 |
16 (0.55) |
146 090 592 |
389 (0.27) |
2.1 (1.2–3.4) |
||||||||||
|
All ages |
6 640 822 |
184 (2.77) |
215 046 031 |
1557 (0.72) |
3.8 (3.3–4.5) |
||||||||||
|
|
|||||||||||||||
|
IR = mean incidence rate: cases per 100 000 person-years. |
|||||||||||||||
Received 23 November 2016, accepted 13 April 2017
- Brett N Archer1
- Clayton K Chiu1,2
- Sanjay H Jayasinghe1,2
- Peter C Richmond3,4,5
- Jodie McVernon6,7,8
- Monica M Lahra9,10,11
- Ross M Andrews12
- Peter B McIntyre1,2
- on behalf of the Australian Technical Advisory Group on Immunisation (ATAGI) Meningococcal Working Party
- 1 National Centre for Immunisation Research and Surveillance (NCIRS), Sydney, NSW
- 2 Sydney Medical School, University of Sydney, Sydney, NSW
- 3 Wesfarmers Centre of Vaccines and Infectious Diseases, Telethon Kids Institute, Perth, WA
- 4 University of Western Australia, Perth, WA
- 5 Princess Margaret Hospital for Children, Perth, WA
- 6 Victorian Infectious Diseases Reference Laboratory, at the Peter Doherty Institute for Infection and Immunity, Melbourne, VIC
- 7 Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC
- 8 Murdoch Children's Research Institute, Melbourne, VIC
- 9 Neisseria Reference Laboratory and WHO Collaborating Centre for Sexually Transmitted Diseases, Prince of Wales Hospital, Sydney, NSW
- 10 South Eastern Area Laboratory Services, Prince of Wales Hospital, Sydney, NSW
- 11 University of New South Wales, Sydney, NSW
- 12 Menzies School of Health Research, Charles Darwin University, Darwin, NT
The members of the Australian Technical Advisory Group on Immunisation (ATAGI) Meningococcal Working Party (2013) and of ATAGI (2013–2014) were Ross Andrews (chair), Michael Nissen, Peter McIntyre, Peter Richmond, Jodie McVernon, Sue Campbell-Lloyd, Karen Peterson, Monica Lahra, Ann Koehler and Julie Leask. We acknowledge the Office of Health Protection (Australian Government, Department of Health and Ageing) and the National Neisseria Network, Australia for providing the data; and all jurisdictional representatives on the National Surveillance Committee (NSC) and the Communicable Diseases Network of Australia (CDNA) for advice on interpreting jurisdiction-specific features of the notified data.
The views expressed in this article are those of its authors, and do not represent the official position of or recommendations by ATAGI or the Australian Government.
- 1. Halperin SA, Bettinger JA, Greenwood B, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine 2012; 30 Suppl 2: B26-B36.
- 2. Jafri RZ, Ali A, Messonnier NE, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr 2013; 11: 17.
- 3. Lahra MM, Enriquez RP. Australian meningococcal surveillance programme annual report, 2015. Commun Dis Intell 2016; 40: E503-E511.
- 4. Sadarangani M, Scheifele DW, Halperin SA, et al. Outcomes of invasive meningococcal disease in adults and children in Canada between 2002 and 2011: a prospective cohort study. Clin Infect Dis 2015; 60: e27-e35.
- 5. Viner RM, Booy R, Johnson H, et al. Outcomes of invasive meningococcal serogroup B disease in children and adolescents (MOSAIC): a case-control study. Lancet Neurol 2013; 11: 774-783.
- 6. Wang B, Afzali HHA, Marshall H. The inpatient costs and hospital service use associated with invasive meningococcal disease in South Australian children. Vaccine 2014; 32: 4791-4798.
- 7. Chiu C, Dey A, Wang H, et al. Vaccine preventable diseases in Australia, 2005 to 2007. Commun Dis Intell Q Rep 2010; 34 Suppl: S1-S167.
- 8. Lawrence G, Wang H, Lahra M, et al. Meningococcal disease epidemiology in Australia 10 years after implementation of a national conjugate meningococcal C immunization programme. Epidemiol Infect 2016; 144: 2382-2391.
- 9. Australian Government, Department of Health. Australian Technical Advisory Group on Immunisation (ATAGI) statement. Advice for immunisation providers regarding the use of Bexsero (March 2014). http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/atagi-advice-bexsero (accessed May 2015).
- 10. Australian Government, Department of Health. Multicomponent meningococcal group B vaccine, 0.5 mL, injection, prefilled syringe, Bexsero® — November 2013 [public summary document]. Pharmaceutical Benefits Scheme; updated Mar 2016. http://www.pbs.gov.au/info/industry/listing/elements/pbac-meetings/psd/2013-11/meningococcal-vaccine (accessed June 2016).
- 11. Pharmaceutical Benefits Scheme (PBS). Recommendations made by the PBAC July 2015: subsequent decisions not to recommend. Pharmaceutical Benefits Scheme; updated Dec 2015. http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/pbac-outcomes/2015-07/web-outcomes-july-2015-subsequent-decision-not-to-recommend.pdf (accessed June 2016).
- 12. Marshall H, Clarke M, Sullivan T. Parental and community acceptance of the benefits and risks associated with meningococcal B vaccines. Vaccine 2014; 32: 338-344.
- 13. Australian Government, Department of Health. Meningococcal disease (invasive) surveillance case definition — V1.4. Updated Jan 2010. http://www.health.gov.au/internet/main/publishing.nsf/Content/cda-surveil-nndss-casedefs-cd_mening.htm (accessed Jan 2014).
- 14. Gibson A, Jorm L, McIntyre P. Using linked birth, notification, hospital and mortality data to examine false-positive meningococcal disease reporting and adjust disease incidence estimates for children in New South Wales, Australia. Epidemiol Infect 2015; 143: 2570-2579.
- 15. Australian Bureau of Statistics. 3101.0. Australian demographic statistics, Jun 2016. Updated 15 Dec 2016. http://www.abs.gov.au/ausstats/abs@.nsf/mf/3101.0 (accessed Mar 2017).
- 16. Australian Bureau of Statistics. 3238.0. Estimates and projections, Aboriginal and Torres Strait Islander Australians, 2001 to 2026. Updated Apr 2014. http://www.abs.gov.au/ausstats/abs@.nsf/mf/3238.0 (accessed May 2015).
- 17. Naidu L, Chiu C, Habig A, et al. Vaccine preventable diseases and vaccination coverage in Aboriginal and Torres Strait Islander people, Australia 2006–2010. Commun Dis Intell Q Rep 2013; 37 (Suppl): S1-S95.
- 18. Borrow R, Abad R, Trotter C, et al. Effectiveness of meningococcal serogroup C vaccine programmes. Vaccine 2013; 31: 4477-4486.
- 19. Andrews SM, Pollard AJ. A vaccine against serogroup B Neisseria meningitidis: dealing with uncertainty. Lancet Infect Dis 2014; 14: 426-434.
- 20. Vesikari T, Esposito S, Prymula R, et al. Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet 2013; 381: 825-835.
- 21. Cohn AC, MacNeil JR, Clark TA, et al. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2013; 62 (RR-2): 1-28.
- 22. MacNeil JR, Bennett N, Farley MM, et al. Epidemiology of infant meningococcal disease in the United States, 2006–2012. Pediatrics 2015; 135: e305-e311.
- 23. Public Health England. Invasive meningococcal disease (laboratory reports in England): 2015/2016 annual data by epidemiological year. Health Protection Report: Weekly Report [online] 2016; 10(37). https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/563949/hpr3716_imd-ann.pdf (accessed July 2017).
- 24. Pollard AJ, Riordan A, Ramsay M. Group B meningococcal vaccine: recommendations for UK use. Lancet 2014; 383: 1103-1104.
- 25. Parikh SR, Andrews NJ, Beebeejaun K, et al. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: a national observational cohort study. Lancet 2016; 388: 2775-2782.
- 26. Martin NV, Ong KS, Howden BP, et al. Rise in invasive serogroup W meningococcal disease in Australia 2013–2015. Commun Dis Intell 2016; 40: E454-E459.
- 27. Government of South Australia, University of Adelaide. B part of it [website]. Updated 28 Nov 2016. http://www.bpartofit.com.au (accessed Mar 2017).
- 28. Novartis Vaccines and Diagnostics. Bexsero® suspension for injection [product information]. 2013; updated July 2017. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2013-PI-02131-1 (accessed Mar 2017).
- 29. Poolman JT, Richmond P. Multivalent meningococcal serogroup B vaccines: challenges in predicting protection and measuring effectiveness. Expert Rev Vaccines 2015; 14: 1277-1287.
- 30. Patel MS. Australia’s century of meningococcal disease: development and the changing ecology of an accidental pathogen. Med J Aust 2007; 186: 136-141. <MJA full text>
- 31. Hart JT. The inverse care law. Lancet 1971; 297: 405-412.
- 32. National Centre for Immunisation Research and Surveillance. Meningococcal disease. Meningococcal vaccines for Australians: information for immunisation providers [fact sheet]. Mar 2017. http://www.ncirs.edu.au/assets/provider_resources/fact-sheets/meningococcal-vaccines-fact-sheet.pdf (accessed Aug 2017).





Abstract
Objectives: To describe trends in the age-specific incidence of serogroup B invasive meningococcal disease (IMD) in Australia, 1999–2015.
Design, setting, participants: Analysis in February 2017 of de-identified notification data from the Australian National Notifiable Diseases Surveillance System of all notifications of IMD in Australia with a recorded diagnosis date during 1999–2015.
Major outcomes: IMD notification rates in Australia, 1999–2015, by age, serogroup, Indigenous status, and region.
Results: The incidence of meningococcal serogroup B (MenB) disease declined progressively from 1.52 cases per 100 000 population in 2001 to 0.47 per 100 000 in 2015. During 2006–2015, MenB accounted for 81% of IMD cases with a known serogroup; its highest incidence was among infants under 12 months of age (11.1 [95% CI, 9.81–12.2] per 100 000), children aged 1–4 years (2.82 [95% CI, 2.52–3.15] per 100 000), and adolescents aged 15–19 years (2.40 [95% CI, 2.16–2.67] per 100 000). Among the 473 infants under 2 years of age with MenB, 43% were under 7 months and 69% under 12 months of age. The incidence of meningococcal serogroup C (MenC) disease prior to the introduction of the MenC vaccine in 2003 was much lower in infants than for MenB (2.60 cases per 100 000), the rate peaking in people aged 15–19 years (3.32 per 100 000); the overall case fatality rate was also higher (MenC, 8%; MenB, 4%). The incidence of MenB disease was significantly higher among Indigenous than non-Indigenous Australians during 2006–2015 (incidence rate ratio [IRR], 3.8; 95% CI, 3.3–4.5).
Conclusions: Based on disease incidence at its current low endemic levels, priority at risk age/population groups for MenB vaccination include all children between 2 months and 5 years of age, Indigenous children under 10 years of age, and all adolescents aged 15–19 years. Given marked variation in meningococcal disease trends over time, close scrutiny of current epidemiologic data is essential.