The known Detailed assessment of patients presenting to emergency departments (EDs) with a suspected acute coronary syndrome (ACS) is inefficient, as most will not have an ACS.
The new The Improved Assessment of Chest pain Trial (IMPACT) protocol maintained clinical safety while reducing the time required for chest pain investigation. It identified low risk patients for whom further objective testing could be safely forgone.
The implications Three-quarters of patients presenting to EDs with chest pain can be rapidly assessed using modified risk stratification criteria, early serial troponin testing, and selective objective testing. Reducing unnecessary objective testing of patients at low risk of an ACS should be encouraged.
Chest pain is among the leading reasons for people to present to an emergency department (ED).1 The 2016 guidelines of the National Heart Foundation of Australia (NHFA) and the Cardiac Society of Australia and New Zealand (CSANZ) for assessing patients with a possible acute coronary syndrome (ACS) recommend electrocardiography (ECG), serial troponin testing, and risk stratification according to an evidence-based suspected ACS assessment protocol.2 Patients deemed to be at high risk of an ACS are referred for admission and investigation; intermediate risk patients require further objective testing for underlying coronary artery disease; and low risk patients can be discharged home. After this often lengthy assessment process, fewer than one in seven patients are diagnosed with an ACS.1
Current risk stratification systems have several limitations. First, the validation of risk scores outside the context of observational trials has been limited.3-6 Second, many novel accelerated strategies focus solely on excluding myocardial infarction, without evidence that they are safe for excluding ACS altogether. Third, existing systems are limited in their ability to identify a significant number of low risk patients who could be safely discharged without further testing. While the 2016 NHFA/CSANZ guidelines identified a small number of patients who may not benefit from objective testing beyond troponin and ECG assessment,2,7 it was also noted that evidence supporting this approach is scarce. International guidelines recommend objective testing to exclude coronary ischaemia,2,8,9 and most patients undergo additional investigation for coronary artery disease. The value of this objective testing for most patients has not been established.10,11
In this article, we describe the Improved Assessment of Chest pain Trial (IMPACT) protocol. This accelerated risk stratification protocol is an alternative to the 2016 NHFA/CSANZ guidelines for patients who present to the ED with a suspected ACS. IMPACT aimed to accelerate the process of assessing low and intermediate risk patients while maintaining their safety; it is based upon the results of sensitive troponin tests 0 and 2 hours after presentation. A key additional focus was to identify a sizeable group of low risk patients who can safely be discharged without further objective testing.
Methods
Study design and setting
IMPACT was a non-randomised intervention trial that included 1366 adult ED patients with possible ACS, recruited prospectively by research staff between 8 am and 5 pm during February 2011 – March 2014. Eligible were consenting patients at least 18 years old who had presented to the ED of the Royal Brisbane and Women’s Hospital, had experienced at least 5 minutes of symptoms suggestive of ACS, and were undergoing assessment for ACS. In accordance with American Heart Association definitions, symptoms suggestive of ACS included acute pain in the chest, epigastrium, neck, jaw or arm, or discomfort or pressure without an apparent non-cardiac cause.12 Patients were excluded if there was a clear non-ACS cause for their symptoms, if they were unwilling or unable to provide informed consent (eg, language barrier), had been transferred from another hospital, were pregnant, had already been recruited to the study in the past 30 days, were unable or unwilling to be contacted after discharge, or if staff considered recruitment inappropriate (eg, in cases of terminal illness).
Interventions
Risk stratification followed the IMPACT protocol (Box 1). Initial troponin and ECG testing was performed on presentation. High risk patients were treated according to the 2006 NHFA/CSANZ guidelines.13 Low and intermediate risk patients were assessed with an accelerated investigation strategy, with repeat troponin testing 2 hours after the first test. Routine inpatient stress testing was recommended only for intermediate risk patients (online Appendix). Low risk patients were discharged if troponin test results were below the 99th percentile of a normal reference population, with a letter to their general practitioner stating that further objective testing was not indicated.
Methods and measurements
Research nurses collected data according to standardised reporting definitions.14 If patients were unsure about an item, a “no” response was recorded unless patients were taking medication for the relevant condition. Troponin was measured with the Beckman–Coulter second generation AccuTnI assay. This sensitive troponin assay has a coefficient of variation (CV) of 14% at the 99th percentile value of 0.04 µg/L, and a 10% CV at 0.06 µg/L.15 Values exceeding 0.040µg/L were deemed to be elevated. Blood samples were collected on presentation and at 2 hours for low and intermediate risk patients, and at 0 and 6 hours for high risk patients. All available troponin results were included in clinical decision making. ECG was performed on presentation and at 2 hours for low and intermediate risk patients, and interpreted by the treating ED clinicians. ECG changes classed as high risk criteria included persistent or dynamic changes of ST-segment depression (= 0.5 mm) or new T-wave inversion (= 2 mm), and transient ST-segment elevation (= 0.5 mm) in more than two contiguous leads. Patients with other abnormal ECG findings (eg, pacing artefact, left bundle branch block) detected by earlier ECG were not defined as high risk patients. Administrative database data were assessed to determine health service use, including ED and hospital length of stay (LOS).
Research nurses followed up patients by telephone 30 days after presentation. All information was verified against medical record databases and cardiac investigation results.
Outcomes
The primary outcome was an ACS within 30 days of presentation, including acute myocardial infarction (AMI), cardiovascular death, unstable angina pectoris (UAP), or coronary revascularisation (emergency or urgent). Type 2 AMIs, infarction secondary to an acute imbalance between oxygen supply and demand (eg, ventricular arrhythmias, heart failure, coronary artery spasm),16 were not included in the primary outcome for this study. The 30-day outcomes were adjudicated independently according to standardised reporting definitions by local cardiologists who had access to the clinical record, ECG and troponin assay results, and all subsequent standard care investigations.13 A second cardiologist conducted a blind review of all ACS and 10% of non-ACS cases. When there was disagreement between the two adjudicators, endpoints were agreed by consensus. Secondary outcomes were ED and hospital LOS.
AMI was diagnosed according to international guidelines, and was based on evidence of myocardial necrosis and ischaemia,16 including ECG and imaging findings. Necrosis was defined as a 20% increase or decrease in cardiac troponin concentration, with at least one value above the 99th percentile of the normal population. Diagnosis of UAP was based on ischaemic symptoms, ECG changes, and objective investigations (exercise stress testing, stress echocardiography, computed tomographic coronary angiography [CTCA], myocardial perfusion scan or angiography) with normal biomarker levels. This included patients with new symptoms or a changing symptom pattern (ie, from stable to unstable angina). Patients with equivocal ECG changes but clear positive changes on exercise testing or imaging evidence of critical coronary stenosis were also classified as having UAP.
Statistical analysis
Baseline demographic data are reported for the entire cohort. Median LOS, the proportion of patients with ACS, and the proportion of patients undergoing objective testing are reported. Data were analysed in Stata 14 (StataCorp).
Ethics approval
The IMPACT study was approved by the Royal Brisbane and Women’s Hospital Human Research and Ethics Committee (reference, HREC/10/QRBW/403). Informed consent was obtained from all participants.
Results
The mean age of the 1366 participants was 50.9 years (standard deviation, 12.8 years), and 819 (60%) were men (Box 2). The IMPACT protocol stratified 244 (17.9%) patients as being at low risk, 789 (57.7%) at intermediate risk, and 333 (24.4%) at high risk of an ACS (Box 2, Box 3). Two patients with diabetes were incorrectly enrolled as low risk patients, as was one patient with an estimated glomerular filtration rate of 59 mL/min/1.73 m2. Nineteen patients were incorrectly enrolled as intermediate risk patients, including five who reported syncope at presentation, nine who were in Killip class II or above, two who had elevated troponin values (0.043 µg/L), and three with ECG abnormalities. All were ultimately diagnosed with non-cardiovascular disease, except for two diagnosed with “other cardiovascular complaints” (pulmonary embolism, syncope of uncertain cause).
Objective testing of 741 low and intermediate risk patients (71.7%) was undertaken (Box 4), including 77 tests (7.5%) in an outpatient setting. The median hospital LOS was 5.1 hours (interquartile range [IQR], 4.2–6.6 h) for low risk and 7.7 hours (IQR, 6.1–21 h) for intermediate risk patients (Box 5).
The overall 30-day ACS rate was 6.6%. There were no cases of ACS in the low risk group and 14 (1.8%) in the intermediate risk group (Box 5). ACS was identified in 13 patients in the intermediate risk group by inpatient objective testing, including eight who had an exercise stress test as their first test. The other five patients underwent other initial testing because of physical inability (one), ECG abnormalities that were not classically ischaemic (one), and clinician decision (three patients). Of the 14 patients with an ACS, one underwent CTCA, three angiography, and one echocardiography. One patient had discharged themselves against medical advice before objective testing, and had an outpatient exercise stress test the next day that indicated ischaemia; they were diagnosed with UAP and therefore classed as a missed case of ACS.
Discussion
The combination of risk stratification, 2-hour serial troponin results, and selected early objective testing for coronary ischaemia according to the IMPACT protocol provided a safe and efficient means for assessing chest pain in the ED. IMPACT reduced the median total chest pain assessment period for the three-quarters of patients stratified as being at low (18%) or intermediate risk (58%), and removed the need for outpatient testing of these patients. Moreover, IMPACT identified a group of low risk patients for whom forgoing objective testing beyond troponin and ECG testing was appropriate and safe.
The IMPACT study is the first large trial to employ widely available diagnostic tools, to implement a strategy of no additional testing for low risk patients, and to report the outcomes. While some of the patients stratified in the low risk group underwent objective testing, no low risk patient was subsequently diagnosed with an ACS. IMPACT thus identified a sizeable group of patients (17.9% of presenting patients) with a negligible 30-day risk of an ACS who could be safely discharged without objective testing.
Because of their observational nature, earlier trials of protocols for identifying low risk patients, including the 2-hour Accelerated Diagnostic protocol to Assess Patients with chest pain Trial (ADAPT),3 HEART,17 and the Emergency Department Assessment of Chest pain Score (EDACS)18 could not inform clinicians about whether there was a need for ongoing investigation. Clinician gestalt (overall clinical judgement) may allow confident discharge of about one-quarter of patients with respect to AMI19, but the safety of this approach has not been tested more broadly for ACS. Diagnostic strategies employing CTCA may identify low risk patients and reduce ED LOS, but it is not universally available, and is associated with increased rates of coronary angiography and revascularisation.20
The IMPACT protocol categorised a large cohort of patients (58%) as being at intermediate risk. The rate of ACS events in this group was about 2%, higher than an acceptable miss rate for adverse cardiac events (under 1%).21 This group of patients requires investigation that identifies an ACS while being efficient and cost-effective. Our study found that expedited assessment, including inpatient objective testing, can safely identify ACS in intermediate risk patients. This strategy is efficient: a subset of patients managed by the IMPACT protocol (previously referred to as the Brisbane protocol) during 2011–2013 spent a median 45 minutes less in the ED and an estimated 26 hours less in hospital overall; estimated hospital costs were $1229 lower than for standard care (NHFA/CSANZ guidelines).22 As hospital and ED LOS are important metrics of operational efficiency, routine adoption of the IMPACT protocol could have important system-wide consequences, including reducing overcrowding in EDs.
IMPACT enabled three-quarters of patients to be rapidly discharged from hospital without further assessment. Few studies have reported such large proportions of patients being eligible for accelerated assessment and discharge without outpatient investigation. While the ADAPT accelerated diagnostic protocol (ADP) identified 20% of patients as low risk in clinical practice, most (79%) underwent further outpatient investigations for coronary artery disease.23 A small randomised trial of the HEART pathway (282 participants) found a 21% increase in early discharges, and objective testing of low risk patients was reduced by 12%;24 non-adherence by providers to the HEART pathway affected 20% of patients.25 A randomised study of the EDACS and ADAPT ADPs found that 41.6% and 30.5% of patients (respectively) were at low risk, but continued to recommend early investigation in an ambulatory setting because of the observational nature of the original trials.18 Similar to IMPACT, these ADPs apply early troponin testing (0 and 2 hours for EDACS and ADAPT; 0 and 3 hours for HEART) and a risk stratification score (EDACS, Thrombolysis In Myocardial Infarction [TIMI], or HEART) to identify low risk patients. However, IMPACT enabled the accelerated assessment of a larger group of patients by applying the approach to both low and intermediate risk patients; those in the intermediate risk group could be discharged within 8 hours without requiring outpatient testing.
A major strength of the IMPACT protocol is that it focuses on a broad range of outcome events, including UAP. Many high quality studies have proposed early rule-out strategies for AMI, including the HEART score and early rule-out AMI biomarker strategies.6,26 Including UAP and urgent revascularisation in the outcome measures is of key interest to ED physicians, whose clinical goal is the safety of discharge for patients at low risk of serious harm. Identifying patients at risk of an AMI is crucial, but identifying UAP is also essential, as assessing and managing this condition may avert an AMI. If patients with UAP are not recognised and discharged early, avoidable harm of clinical and medico-legal importance may ensue.
The prevalence of ACS in our high risk group was similar (23%) to that previously reported (28%);27 the risk criteria for this category were unchanged in the new protocol. Irrespective of strategies, patients with high risk features, including elevated troponin levels, are unlikely to be discharged from the ED. It is predominately low and intermediate risk patients managed without comprehensive inpatient assessment who benefit from an accelerated assessment strategy.
Finally, the IMPACT protocol included a sensitive (but not highly sensitive) troponin assay. Highly sensitive assays have lower detection limits and greater precision, perhaps enabling earlier detection of an AMI, but many clinicians do not have access to such assays. Despite highly sensitive troponin assays, the clinical entity of UAP continues to exist,28 and objective testing will still be needed for intermediate risk patients. IMPACT is therefore relevant and safe whether using sensitive or highly sensitive assays.
Limitations
As our study was conducted at a single centre and was not randomised, no comparison data are available. Because of the major change in assessment processes prescribed by the IMPACT protocol, crossover within a randomised intervention would be inevitable. Patients were recruited only during normal working hours, but we have previously reported that the characteristics of patients presenting during and outside 9 am–5 pm do not significantly differ.29 The proportion of patients with a final diagnosis of AMI may be lower than would be identified by a highly sensitive troponin assay. The main objective tests for defining coronary insufficiency in patients in the low and intermediate risk groups were exercise stress tests; while they may not be as diagnostically accurate for detecting coronary artery disease, discharging patients with normal findings from this low risk, inexpensive and widely available investigation is safe.9,30 High risk patients were classified according to the 2006 NHFA/CSANZ guidelines as these were current at the time of the study. The updated guidelines published in 2016 added prior AMI as a high risk feature (the 2006 guidelines included only prior percutaneous coronary intervention or coronary artery bypass graft) and a slightly modified definition of chest pain (as ongoing or repetitive chest pain despite initial ED treatment); they also omitted creatine kinase-MB levels from the definition of elevated biomarkers. Finally, the IMPACT protocol strategy needs further validation to assess its generalisability.
Conclusion
The IMPACT protocol maintained clinical safety and reduced the length of both the ED and hospital periods of evaluation for more than three-quarters of all patients presenting to the ED with symptoms of possible ACS, obviating the need for additional outpatient testing and identifying low risk patients for whom forgoing further objective testing was appropriate and safe.
Box 1 – Risk stratification criteria from the 2006 National Heart Foundation of Australia/Cardiac Society of Australia and New Zealand (NHFA/CSANZ) guidelines and IMPACT protocols
|
2006 NHFA/CSANZ guidelines13 |
IMPACT criteria |
||||||||||||||
|
|
|||||||||||||||
|
High risk |
|
||||||||||||||
|
|
Same as 2006 NHFA/CSANZ guidelines |
||||||||||||||
|
Intermediate risk |
|
||||||||||||||
|
No high risk features, and any of:
|
No high risk features, and any of:
|
||||||||||||||
|
Low risk |
|
||||||||||||||
|
No intermediate or high risk features, and any of:
|
No intermediate or high risk features, and:
|
||||||||||||||
|
|
|||||||||||||||
|
ACS = acute coronary syndrome; CABG = coronary artery bypass graft; CK-MB = creatine kinase-MB; eGFR = estimated glomerular filtration rate; LVEF = left ventricular ejection fraction; PCI = percutaneous coronary intervention. |
|||||||||||||||
Box 2 – Demographic characteristics of the IMPACT participants, overall and by IMPACT risk group
|
|
All patients |
IMPACT risk stratification |
|||||||||||||
|
Low risk |
Intermediate risk |
High risk |
|||||||||||||
|
|
|||||||||||||||
|
Number of patients (% of all patients) |
1366 |
244 (17.9%) |
789 (57.7%) |
333 (24.4%) |
|||||||||||
|
Age (years), mean (SD) |
50.9 (12.8) |
33.8 (4.4) |
52.8 (8.9) |
59.1 (13.6) |
|||||||||||
|
Age range (years) |
20–95 |
20–39 |
40–87 |
26–95 |
|||||||||||
|
Sex (men) |
819 (60.0%) |
163 (66.8%) |
411 (52.1%) |
245 (73.6%) |
|||||||||||
|
Risk factors |
|
|
|
|
|||||||||||
|
Hypertension |
534 (39.1%) |
27 (11%) |
273 (34.6%) |
234 (70.3%) |
|||||||||||
|
Dyslipidaemia |
510 (37.3%) |
20 (8.2%) |
270 (34.2%) |
220 (66.1%) |
|||||||||||
|
Diabetes |
161 (11.8%) |
2 (0.8%) |
42 (5.3%) |
117 (34.1%) |
|||||||||||
|
Family history of coronary artery disease |
499 (36.5%) |
74 (30%) |
282 (35.7%) |
143 (42.9%) |
|||||||||||
|
Current or recent smoking |
385 (28.2%) |
91 (37%) |
209 (26.5%) |
85 (26%) |
|||||||||||
|
Medical history |
|
|
|
|
|||||||||||
|
Myocardial infarction |
157 (11.5%) |
1 (0.4%) |
39 (4.9%) |
117 (35.1%) |
|||||||||||
|
Angina |
148 (10.8%) |
2 (0.8%) |
38 (4.8%) |
108 (32.4%) |
|||||||||||
|
Coronary artery bypass graft |
50 (3.7%) |
0 |
0 |
50 (15%) |
|||||||||||
|
Angioplasty |
101 (7.4%) |
0 |
26 (3.3%) |
75 (22%) |
|||||||||||
|
Risk stratification after 2-hour troponin testing, according to 2006 NHFA/CSANZ guidelines |
|
|
|
||||||||||||
|
Low risk |
14 (1.0%) |
14 (5.7%) |
0 |
0 |
|||||||||||
|
Intermediate risk |
1000 (73.2%) |
230 (94.3%) |
770 (97.6%) |
0 |
|||||||||||
|
High risk |
352 (25.8%) |
0 |
19 (2.4%) |
333 (100.0%) |
|||||||||||
|
|
|||||||||||||||
|
NHF/CSANZ = National Heart Foundation/Cardiac Society of Australia and New Zealand. |
|||||||||||||||
Box 3 – Patient flow through the IMPACT accelerated assessment pathway, including protocol deviations
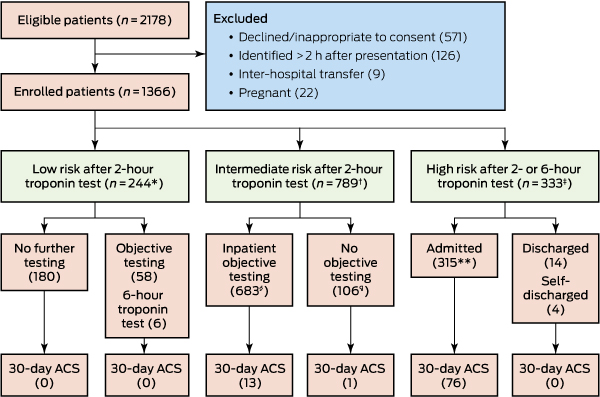
* Two patients did not undergo 2-hour troponin testing; 36 did not undergo 2-hour electrocardiography (ECG). † Five patients did not undergo 2-hour troponin testing; 76 did not undergo 2-hour ECG. ‡ 44 patients did not undergo 6-hour troponin testing; 30 did not undergo 2-hour ECG. § For 51 patients, the results of the objective test were unremarkable but 6-hour troponin testing was also undertaken; 11 underwent further objective testing after an unremarkable objective test without undergoing 6-hour testing. Two patients discharged themselves without sufficient clinical information for determining the endpoint. ¶ Ten patients discharged themselves without sufficient clinical information for determining the endpoint. Two patients were discharged after equivocal exercise stress test results, and eight discharged themselves before objective testing. Each of these patients was followed up by telephone and in the National Death Index; none re-presented to hospital, visited their general practitioner, or had died at 12 months, so they were classed as not having had an ACS. ** One patient discharged themselves without sufficient clinical information for determining the endpoint. This patient had not re-presented to hospital, visited their general practitioner, or died at 12 months, so they were classed as not having had an ACS.
Box 4 – Inpatient objective testing of 1033 low and intermediate risk patients*
|
|
|||||||||||||||
|
Any inpatient test |
741 (71.7%) |
||||||||||||||
|
Exercise stress test |
681 (65.9%) |
||||||||||||||
|
Myocardial perfusion scintigraphy |
29 (2.8%) |
||||||||||||||
|
Stress echo |
7 (0.7%) |
||||||||||||||
|
Computed tomographic coronary angiography |
26 (2.5%) |
||||||||||||||
|
Echocardiography |
81 (7.8%) |
||||||||||||||
|
Angiography |
60 (5.8%) |
||||||||||||||
|
|
|||||||||||||||
|
* Patients could undergo more than one test. |
|||||||||||||||
Box 5 – Primary and secondary outcomes for the IMPACT participants, by risk category
|
|
All patients |
IMPACT risk stratification |
|||||||||||||
|
Low risk |
Intermediate risk |
High risk |
|||||||||||||
|
|
|||||||||||||||
|
Number of patients |
1366 |
244 |
789 |
333 |
|||||||||||
|
30-day acute coronary syndrome events (including index events)* |
90 (6.6%) |
0 |
14 (1.8%) |
76 (23%) |
|||||||||||
|
Number of index ACS cases not diagnosed in hospital (missed cases) |
1 (0.1%) |
0 |
1 (0.1%) |
0 |
|||||||||||
|
One-year mortality (for patients who could be followed up) |
13 of 1160 (1.1%) |
0 of 200 |
2 of 683 (0.3%) |
11 of 277 (4.0%) |
|||||||||||
|
Emergency department length of stay (hours), median (IQR) |
3.2 |
3.2 |
2.8 |
5.0 |
|||||||||||
|
Hospital length of stay (hours), median (IQR) |
8.2 |
5.1 |
7.7 |
49.4 |
|||||||||||
|
|
|||||||||||||||
|
ACS = acute coronary syndrome. * All were index acute coronary syndrome events. |
|||||||||||||||
Received 25 November 2016, accepted 21 March 2017





Abstract
Objective: To examine the safety and efficacy of the Improved Assessment of Chest pain Trial (IMPACT) protocol, a strategy for accelerated assessment of patients presenting to emergency departments (EDs) with chest pain.
Design, setting and participants: IMPACT was an intervention trial at a single tertiary referral hospital (Royal Brisbane and Women’s Hospital) during February 2011 – March 2014. 1366 prospectively recruited patients presenting to the ED with symptoms of suspected acute coronary syndrome (ACS) were stratified into groups at low, intermediate or high risk of an ACS.
Intervention: High risk patients were treated according to NHFA/CSANZ guidelines. Low and intermediate risk patients underwent troponin testing (sensitive assay) 0 and 2 hours after presentation. Intermediate risk patients underwent objective testing after the second troponin test; low risk patients were discharged without further objective testing.
Main outcome measures: The primary outcome was an ACS within 30 days of presentation. Secondary outcomes were ED and hospital lengths of stay (LOS).
Results: The IMPACT protocol stratified 244 (17.9%) patients to low risk, 789 (57.7%) to intermediate risk, and 333 (24.4%) to high risk categories. The overall 30-day ACS rate was 6.6%, but there were no ACS events in the low risk group, and 14 (1.8%) in the intermediate risk group. The median hospital LOS was 5.1 hours (IQR, 4.2–5.6 h) for low risk and 7.7 hours (IQR, 6.1–21 h) for intermediate risk patients.
Conclusions: The IMPACT protocol safely and efficiently allowed a large proportion of patients presenting to EDs with chest pain to undergo accelerated assessment for risk of an ACS.
Clinical trial registration: Australian New Zealand Clinical Trials Registry ACTRN12611000206921.