Clostridium difficile was first isolated in 1935,1,2 but was probably responsible for outbreaks of diarrhoea for centuries before its discovery.3 C. difficile-associated diarrhoea (CDAD) was until recently an uncommon disorder that usually responded to antibiotics and rarely life-threatening. Over the past 20 years, the worldwide incidence of CDAD has more than doubled,4 and outbreaks have been associated with greater morbidity and mortality,5 although to a lesser extent in Australia.6 In-hospital mortality directly attributed to the infection has been estimated to be 5%, and all-cause mortality at greater than 15%.5
Genotyping of C. difficile in the early 2000s revealed that a particularly pathogenic strain (BI/NAP1/027) has an 18-base pair deletion in the tcdC gene, resulting in increased antibiotic resistance and a more than tenfold increase in the production of both types of toxin released by the bacterium.7 The emergence of a more toxic, antibiotic-resistant strain of C. difficile is a major problem, particularly for vulnerable hospitalised patients; in 2011, C. difficile infection was associated with more than 450 000 inpatient cases in the United States, causing almost 30 000 deaths and incurring costs of US$1.5 billion.8
Faecal microbiota transplantation (FMT) has emerged as an important approach to treating CDAD resistant to antibiotic therapy. Treating patients with abdominal disorders with faecal preparations was first described more than 1000 years ago,9 but only recently has its potential value for treating CDAD been realised.10 Recent guidelines from Europe11,12 and North America13 recommend FMT for treating antibiotic-resistant CDAD; Australian and New Zealand guidelines on therapy for CDAD need to be updated.14 However, the overseas guidelines are based on systematic reviews of data largely derived from case series,13,15 so that the quality of evidence is not optimal. Several randomised controlled trials (RCTs) of FMT for CDAD have since been published, motivating us to undertake a systematic review of these RCTs that could inform future guidelines on the topic.
Methods
Search strategy and study selection
We undertook a literature search in the electronic databases MEDLINE (1946 to 6 February 2017), EMBASE (1947 to 6 February 2017), the Cochrane Central Register of Controlled Trials (to November 2016), and the Cochrane Database of Systematic Reviews (2005 to 2 February 2017). We included RCTs that evaluated FMT in adult patients (over the age of 16 years) with CDAD (Box 1). Studies of CDAD were identified by the term Clostridium difficile (as a medical subject heading [MeSH] or free text) combined (set operator AND) with studies identified by “faecal microbiota transplantation” (MeSH and free text), “fecal/faecal adj5 transplant”, “fecal/faecal adj5 therapy”, “bacteriotherapy”, or similar terms (online Appendix 1).
Abstracts were eligible for inclusion; we hand-searched conference proceedings for United European Gastroenterology Week and for Digestive Disease Week in the US and Canada for 2015 and 2016. ClinicalTrials.gov was searched for ongoing trials of FMT for C. difficile infections, and we also conducted a recursive search of the literature in the bibliographies of relevant studies identified by our search strategy. Two masked reviewers (YY and PM) independently assessed potentially relevant articles according to prospectively defined eligibility criteria (Box 1). Any disagreement between investigators was resolved by consensus (all four authors).
Population, intervention, controls, outcome assessment
The primary outcome assessed was resolution of CDAD symptoms as defined by the trial investigators. Where more than one definition of success was applied, we chose the outcome with the lowest placebo response rate. Secondary outcomes assessed included quality of life and adverse events.
Data extraction
Two reviewers (PM and YY) independently recorded data from eligible studies in an Excel (Microsoft) spreadsheet. Reviewers extracted the following information for each trial: setting (primary, secondary, or tertiary care-based), number of centres, country of origin, proportion of female patients, type of FMT donor, route of administration, use of bowel preparation or antibiotics prior to FMT, number of FMT doses, primary outcome, total number of adverse events reported, quality of life, and duration of follow-up. Data were extracted for intention-to-treat analyses, with all drop-outs assumed to be treatment failures. Two independent reviewers (PM and YY) also assessed the risk of bias according to the Cochrane handbook risk-of-bias tool.16 This evaluates the method of randomisation, whether allocation was concealed, the method of blinding, the completeness of follow-up, evidence of selective outcome reporting, and other biases.
Data synthesis and statistical analysis
The relative proportions of patients with persistent CDAD in the intervention and control groups were expressed as a relative risk (RR) with 95% confidence intervals (CIs). The relative frequencies of adverse events were also expressed as RRs with 95% CIs. Data were pooled in a random effects model17 to allow for any heterogeneity across studies. The number needed to treat (NNT) and the number needed to harm (NNH), with 95% CIs, were calculated from the meta-analysis RR and assumed control group risk (ACR), based on the pooled control event rate from the eligible studies: NNT/NNH = 1/(ACR × [1 – RR]).
Heterogeneity of studies was assessed with both the I2 statistic (cut-off, ≥ 50%) and the χ2 test (P < 0.10 defined as significant degree of heterogeneity).18 If heterogeneity was significant, we explored potential explanations in planned subgroup analyses19 according to type of control, FMT route, number of doses, trial setting, criteria for success, and risk of trial bias. We compared individual RRs from these analyses in the Cochran Q statistic (χ2 test).
Forest plots of pooled RRs for primary and secondary outcomes with 95% CIs and funnel plots were generated in Review Manager 5.3.5 (RevMan; the Nordic Cochrane Centre). Had more than nine eligible trials been synthesised,20 a funnel plot would have been generated to assess asymmetry, and hence possible publication bias or other small study effects, with the Egger test.21
Results
The search strategy identified 309 relevant articles, of which ten reported eligible RCTs that evaluated the treatment of 657 CDAD patients22-31 (Box 2; Box 3). The ten RCTs evaluated FMT for recurrent CDAD that had not responded to, or had recurred after at least one course of antibiotics; most trials defined cure of CDAD as resolution of symptoms. The trials applied different comparators, and evaluated different modes of FMT (Box 3). The risk of bias was low for four trials24,25,30,31 (Box 4).
Comparison of FMT with vancomycin or placebo
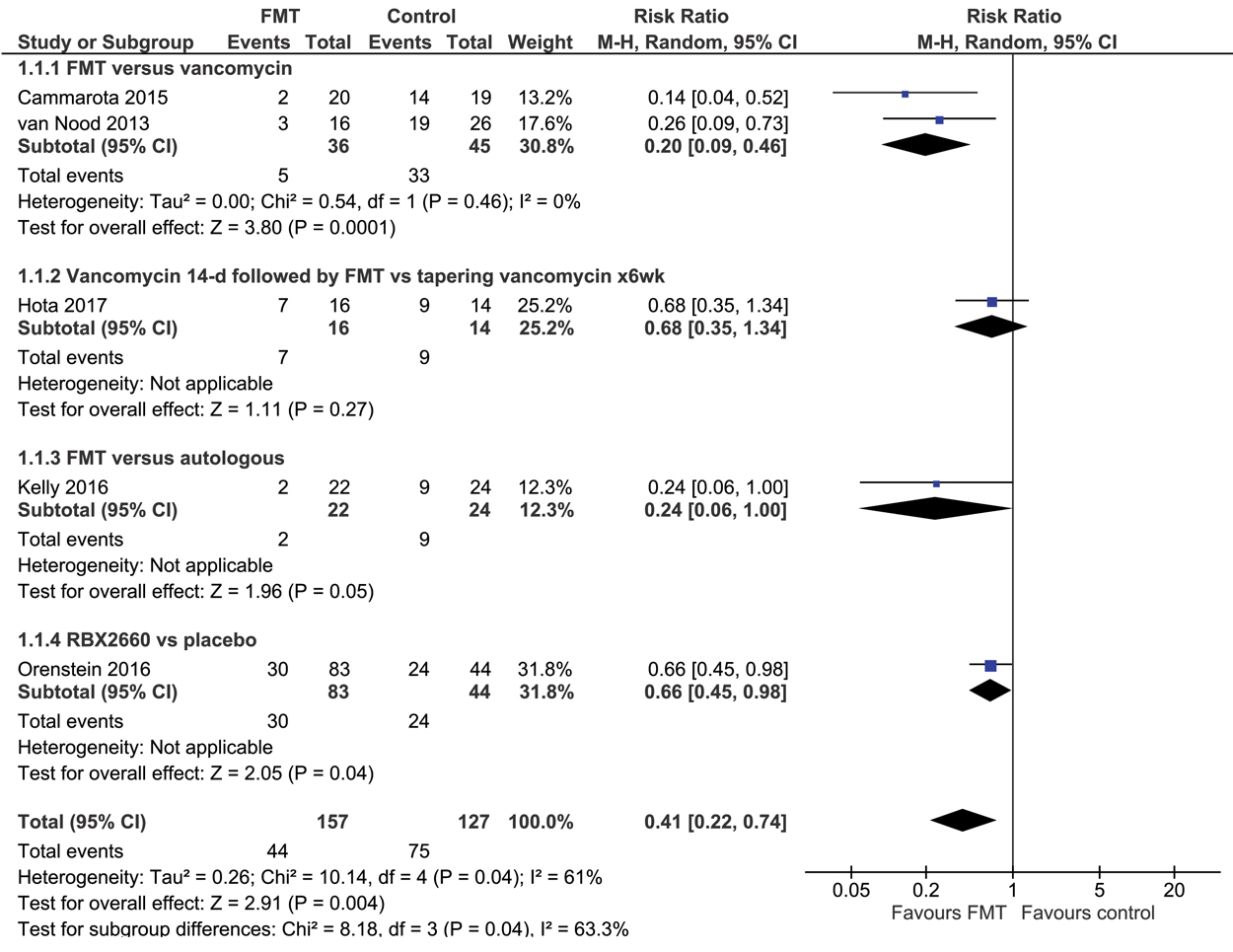
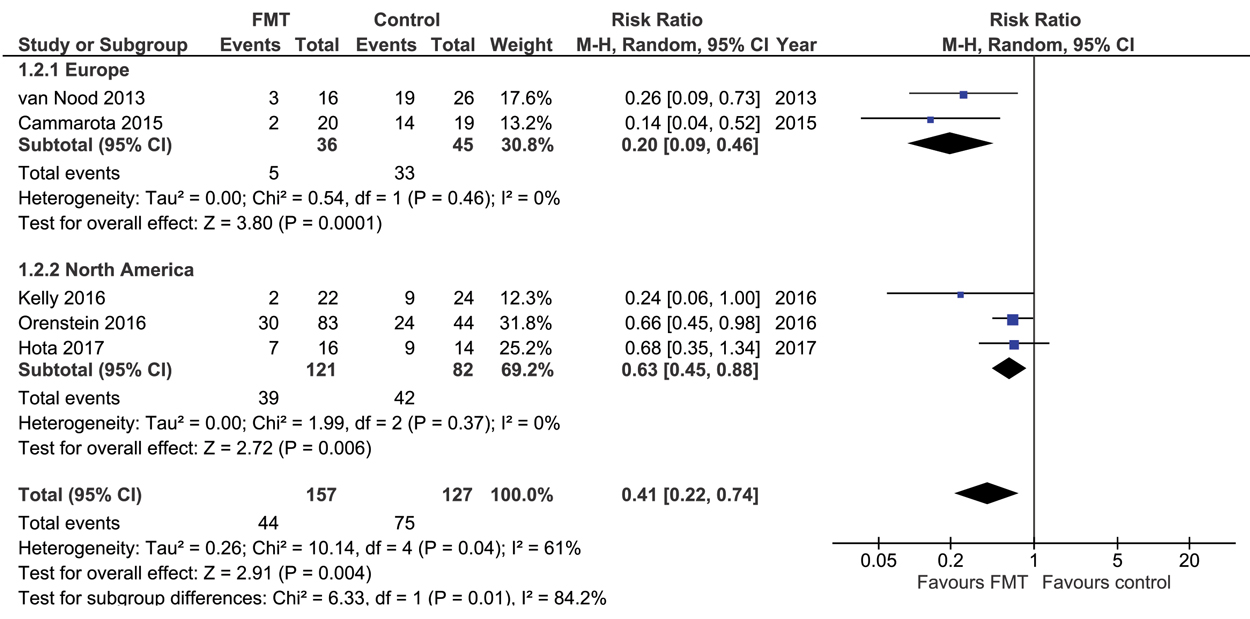
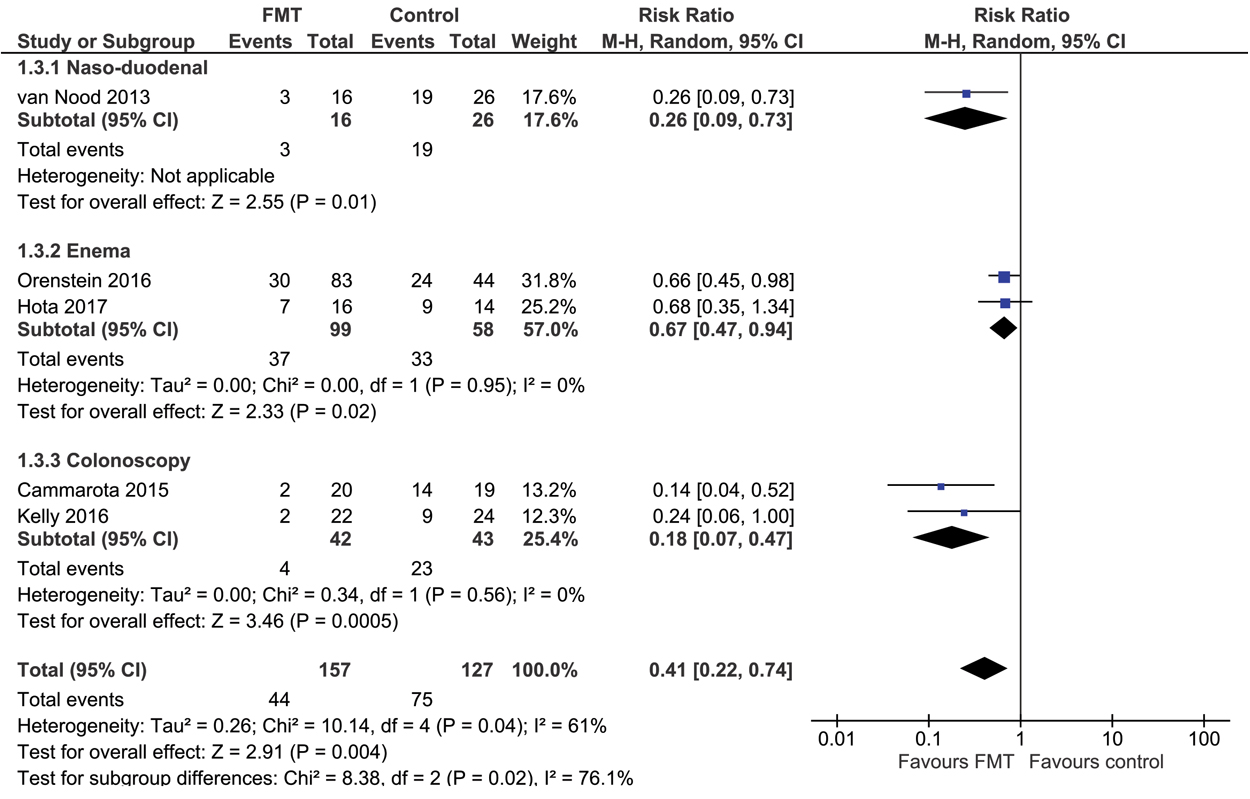
Five RCTs22-26 compared FMT with vancomycin or placebo (total of 284 CDAD patients); in these trials, FMT was statistically significantly more effective in curing CDAD (RR for CDAD persisting, 0.41; 95% CI, 0.22–0.74; P = 0.004; Box 5), with an NNT of 3 (95% CI, 2–7). There was significant heterogeneity across studies (I2 = 61%; χ2 test, P = 0.04). Pre-defined subgroup analyses indicated that the effect of FMT did not differ significantly between trials with low/unclear or high risk of bias, or between trials in which vancomycin or autologous stool/placebo served as control; these factors did not explain the heterogeneity (Box 6). FMT was more effective in European than in North American trials (Box 7), and when administered by the naso-duodenal or colonoscopy routes rather than by enema (Box 8). These two factors explained the heterogeneity among the studies (Box 6), but this finding should be received with caution, as the numbers of studies and patients enrolled were small. There was no difference between FMT and vancomycin or placebo treatments in the number of serious adverse events (RR, 0.64; 95% CI, 0.26–1.61), and there was no heterogeneity across studies in this respect (I2 = 0%; χ2 test, P = 0.64; online Appendix 2, figure). There were no major differences between the FMT and control groups with regard to serious adverse events (online Appendix 2, table).
The quality of the evidence, as assessed according to GRADE criteria,32 was moderate. Although the effect of FMT was statistically significant, the quality of evidence was reduced by the heterogeneity of the studies, both statistical and clinical. The number of events in the trials was also low by GRADE criteria, but we did not downgrade for this factor because the effect of FMT was marked; even at the upper limit of the 95% CI for its relative efficacy (0.74), its effect would be clinically important.
Comparing preparations and routes of delivery of FMT
Five eligible RCTs27-31 compared different preparations and routes of administration of FMT. One trial28 with 19 CDAD patients found no statistically significant difference between the effect of giving 30 FMT capsules once and repeating the dose the next day (cure rates, 70% and 77% respectively). Another trial29 randomised 43 participants to FMT by capsule or by colonoscopy; the cure rates were not statistically different (92% v 100%). A third trial27 compared frozen/thawed FMT administered by nasogastric tube with FMT by colonoscopy in 20 CDAD patients; the cure rates were not statistically different (60% v 80%). Each of these studies was underpowered for detecting meaningful clinical differences between the groups, and all were judged as being at high risk of bias (Box 4). The quality of evidence (GRADE) for each of these comparisons was very low.31
One RCT30 compared frozen with freshly prepared FMT in a trial with 219 CDAD patients. There was no statistically significant difference in the clinical resolution of symptoms (84% and 85% of patients respectively). This study was adequately powered for detecting a difference, and was at low risk of bias (Box 4); however, the quality of evidence for frozen FMT being as effective as fresh FMT was rated as moderate because the number of patients, while reasonably large, was not sufficient to merit a high quality grading. One RCT31 compared fresh, frozen, and lyophilised FMT in a total of 72 CDAD patients, with resolution of CDAD in 100%, 83%, and 78% respectively. There was no statistically significant difference between the effectiveness of fresh and frozen FMT, but fresh FMT was significantly superior to the lyophilised preparation; however, the authors do not appear to have adjusted their analysis for multiple testing. The study sample size was insufficient to provide robust results, and there was an unclear risk of bias (Box 4).
Discussion
A number of systematic reviews have evaluated studies of FMT in patients with CDAD,13,15,33-35 but all have focused on case series data. These reviews all found that FMT was effective for treating CDAD, but uncertainty was inevitable given the low quality of most of the studies assessed. Ours is the first systematic review to focus on RCT evidence for the efficacy of FMT in the treatment of CDAD. Our review more accurately analyses the importance of the route of administration and the choice of control intervention, and we also evaluated the quality of the reported evidence with a robust methodology.32
We found that there is moderate quality evidence that FMT is effective for treating patients with CDAD that has not responded to or has recurred after antibiotic therapy. According to GRADE criteria,32 we can be reasonably confident that FMT is effective, but further trials may change our estimate of the magnitude of its effect. All included trials were undertaken in Europe or North America; RCT data from Australia, New Zealand and Asia would be useful, particularly as the revival of interest in the therapeutic benefit of FMT for patients with gastrointestinal diseases originated in Australia.36
Our systematic review also highlights the fact that frozen/thawed transplants — a more convenient approach that reduces the burden on a donor to supply a sample on the day it is needed — is as effective as fresh FMT. The RCT reporting their similar efficacy30 was supported by a microbiological analysis which found that the viability of six representative bacterial groups changed little during 6 months’ storage at –80°C.37 Lyophilised samples allow easier storage, and also provide material that is simpler to encapsulate.38 However, preliminary RCT findings31 suggest that this approach may not be as efficacious as fresh FMT, and the question needs further investigation. An RCT comparing lyophilised and fresh FMT administered by retention enema is underway,39 but, as only 50 CDAD patients will be recruited, it may have insufficient power to test the equivalence of the two preparations.
Our systematic review has some limitations. We reviewed conference abstracts only from the past two years, and may have missed earlier trials not published as full articles. We did not assess the grey literature, nor did we contact new companies that may be assessing the effectiveness of commercial FMT preparations for treating CDAD. We identified more relevant RCTs than previous reviews, but our conclusions are still limited by the quality of the reported data. In particular, the preparations of FMT evaluated and the nature of the control groups varied between trials. As ethical considerations dictate that patients in the control arm of an RCT receive standard care, treatment with vancomycin or fidaxomicin40 would be appropriate for comparative purposes.11
Further investigations into the best route of administration for FMT are needed. Our analysis indicates that naso-duodenal and colonoscopic application may be more effective than retention enemas, but this conclusion relies on indirect comparisons of subgroups. Further, these modes are difficult to employ more than once in an individual patient, some of whom may require a second FMT. Naso-duodenal application entails the risk of aspiration,41 and colonoscopy a small risk of perforation, which is more significant in patients with severe disease. It is therefore important that an adequately powered RCT compares the efficacy of FMT by enema with that by colonoscopy. Data from RCTs on which type of donor (related, unrelated, or anonymous) is most efficacious have not been published; whether pooling stool from several donors increases the efficacy of FMT, and whether bowel preparation (treatment with polyethylene glycol or antibiotics) improves outcomes also remain to be clarified. More detailed epidemiological data on factors that potentially predict the success or failure of FMT are also needed.
In conclusion, our systematic review synthesises information from RCTs that have evaluated the efficacy of FMT for treating CDAD, and this synthesis will be useful when developing guidelines. It also provides researchers with further information on how to best design RCTs for assessing FMT in patients with CDAD.
Box 1 – Eligibility criteria for inclusion of randomised control trials in our analysis
|
|
|||||||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
|
* Defined as transfer of stool from a healthy donor. Administration of selected bacterial strains (such as “synthetic stool”, probiotics, spore-forming organisms) were excluded by this definition. † Autologous FMT uses stool collected from the patient, processed as donor stool, and administered to the same patient. |
|||||||||||||||
Box 2 – Randomised control trials (RCTs) of faecal microbiota transplantation for treating Clostridium difficile-associated diarrhoea: selection of studies included in the analysis
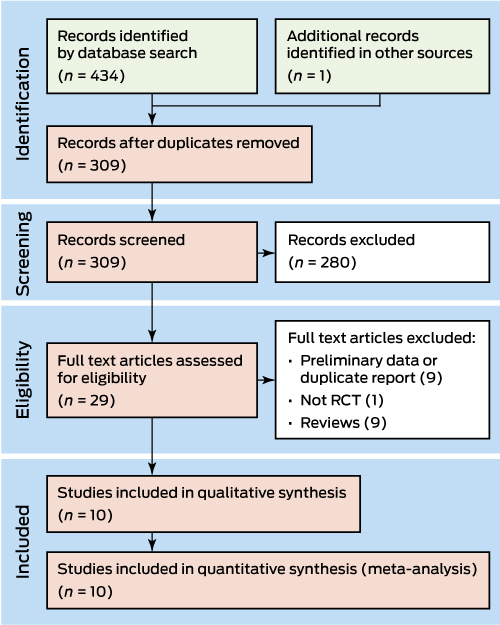
Box 3 – Demographic information, follow-up and outcomes of eligible randomised control trials of faecal microbiota transplantation (FMT) for treating Clostridium difficile-associated diarrhoea (CDAD)
|
Trial |
Country (number of sites) |
Patient characteristics |
Follow-up |
Definition of success |
Intervention |
Donor |
Delivery mode |
Comparator |
|||||||
|
|
|||||||||||||||
|
Trials comparing FMT with placebo or vancomycin therapy |
|||||||||||||||
|
van Nood et al. (2013)22 |
Netherlands (1) |
CDAD recurrence after at least one course of adequate antibiotic therapy (vancomycin: ≥ 125 mg qid, ≥ 10 days; metronidazole: 500 mg tid, ≥ 10 days); n = 42 |
10 weeks |
Absence of diarrhoea, or persistent diarrhoea explicable by other causes, with three consecutive negative stool tests for C. difficile toxin |
Vancomycin (500 mg qid, 4 days), followed by bowel lavage with 4 L macrogol solution (Klean-Prep) on final day of antibiotic treatment and subsequent infusion of a solution of FMT; n = 16 |
Healthy volunteer |
Naso-duodenal tube |
Vancomycin (500 mg qid, 14 days), or vancomycin with bowel lavage on day 4 or 5; n = 26 |
|||||||
|
Cammarota et al. (2015)23 |
Italy (1) |
Recurrent CDAD; n = 39 |
10 weeks |
Disappearance of diarrhoea, or persistent diarrhoea explicable by other causes, with two negative stool tests for C. difficile toxin |
Vancomycin (125 mg qid, 3 days), followed by one or more infusions of faeces (diluted in 500 mL sterile saline); n = 20 |
Healthy volunteer |
Colonoscopy |
Vancomycin (125 mg qid, 10 days), followed by 125–500 mg/day every 2–3 days for at least 3 weeks; n = 19 |
|||||||
|
Kelly et al. (2016)24 |
USA (2) |
Three or more CDAD recurrences; course of vancomycin received; n = 46 |
8 weeks |
Resolution of diarrhoea without need for further anti-CDAD therapy for 8 weeks |
300 mL faecal suspension infused into terminal ileum or caecum; n = 22 |
Healthy volunteer (identified by patients or anonymous) |
Colonoscopy |
Autologous FMT: 300 mL faecal suspension from own stool infused into terminal ileum or cecum; n = 24 |
|||||||
|
Orenstein et al. (2016)25 |
USA/Canada (21) |
At least two CDAD recurrences; n = 127 |
13 weeks |
Clinical resolution of diarrhoea without relapse at 13 weeks, after receiving up to two FMTs without need for antibiotics |
RBX2660: one or two doses, 7 days apart; n = 83 |
Commercially prepared microbiota suspension |
Enema |
Two doses of placebo, 7 days apart; n = 44 |
|||||||
|
Hota et al. (2017)26 |
Canada (1) |
Two or more CDAD recurrences; at least one course of oral vancomycin received; n = 30 |
120 days |
No recurrence of CDAD within 120 days (primary) or no recurrence of CDAD symptoms (not laboratory-confirmed) (secondary outcome) |
Oral vancomycin (125 mg qid, 14 days) followed by single 500 mL FMT; n = 16 |
16 fresh donations from screened, healthy donors identified by recipients |
Enema |
Oral tapering vancomycin for 6 weeks: 125 mg, qid, 14 days; then twice daily, once daily, every second day, every third day: each for a week; n = 14 |
|||||||
|
Trials comparing FMT modalities |
|||||||||||||||
|
Youngster et al. (2014)27 |
USA (1) |
At least three episodes of mild to moderate CDAD and failure of 6–8 week taper with vancomycin; or at least two episodes of severe CDAD resulting in hospitalisation, associated with significant morbidity; n = 20 |
6 months |
Clinical resolution of diarrhoea without relapse at 8 weeks |
41 g stool sample diluted in sterile saline. Frozen suspensions were stored (−80°C) for maximum 156 days, thawed in 37°C water bath; n = 10 |
Unrelated healthy donor |
FMT via colonoscopy |
FMT: By nasogastric tube (omeprazole 20 mg daily, 48 hours before infusion); n = 10 |
|||||||
|
Allegretti et al. (2016)28 |
USA (2) |
Recurrent CDAD; n = 19 |
8 weeks |
Clinical resolution of diarrhoea without relapse at 8 weeks |
30 FMT capsules; n = 9 |
Healthy, rigorously screened, unrelated donors from a universal public stool bank |
Oral capsule |
FMT: 30 FMT capsules given on two consecutive days; n = 10 |
|||||||
|
Kao et al. (2016)29 |
Canada (2) |
Three or more CDAD recurrences; n = 43 |
Not reported |
Not defined |
100 g stool processed to 40–60 capsules; n = 22 |
Seven universal stool donors registered in FMT program |
Oral capsule |
FMT: By colonoscopy: 100 g raw stool processed to 400 mL faecal slurry for colonoscopy delivery; n = 21 |
|||||||
|
Lee et al. (2016)30 |
Canada (6) |
History of recurrent or refractory CDAD; patients with only one recurrence were not included unless the most recent episode was refractory to treatment; n = 219 |
13 weeks |
Clinical resolution of diarrhoea without relapse at 13 weeks, after receiving up to two FMTs without need for antibiotics |
100 g stool sample diluted in 300 mL water. Frozen suspensions were stored (−20°C) for maximum 30 days, thawed overnight (25°C); suspension administered within 24 hours; n = 108 |
Unrelated healthy volunteers (most supplied by three donors) |
Enema |
FMT: Fresh 100 g stool sample diluted with 300 mL water; patients received suspension within 24 hours; n = 111 |
|||||||
|
Jiang et al. (2017)31 |
USA (1) |
At least three separate episodes of CDAD in past 3 months; n = 72 |
5 months |
Resolution of symptoms and no recurrence during 5 months’ follow-up |
Individual stool samples from donors (≥ 50 g) processed within 4 hours of passage, diluted in 0.85% saline (total volume, 1500 mL). Fresh FMT aliquots were administered within 2 hours of preparation; n = 25 |
Unrelated healthy donor |
Colonoscopy |
FMT: Frozen or lyophilised |
|||||||
|
|
|||||||||||||||
|
qid = four times a day; tid = three times a day. |
|||||||||||||||
Box 4 – Summary of the risk of bias for each included randomised control trial of faecal microbiota transplantation (FMT)
|
|
Trials comparing FMT with placebo or vancomycin therapy* |
Trials comparing FMT modalities* |
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|||||||||||||||
|
Random sequence generation (selection bias) |
? |
— |
— |
? |
? |
— |
? |
? |
— |
— |
|||||
|
Allocation concealment (selection bias) |
? |
? |
? |
— |
? |
? |
? |
? |
— |
? |
|||||
|
Blinding of participants and personnel (performance bias) |
X |
X |
— |
— |
X |
X |
? |
? |
— |
— |
|||||
|
Blinding of outcome assessment (detection bias) |
? |
? |
— |
— |
? |
? |
? |
? |
— |
— |
|||||
|
Incomplete outcome data (attrition bias) |
— |
— |
— |
— |
— |
— |
— |
— |
— |
— |
|||||
|
Selective reporting (reporting bias) |
— |
— |
— |
— |
— |
— |
— |
— |
— |
— |
|||||
|
Other bias |
? |
? |
— |
— |
? |
— |
? |
? |
— |
— |
|||||
|
|
|||||||||||||||
|
* The studies are identified by the number of the publication in the reference list. — = low risk of bias; ? = unclear risk of bias; X = high risk of bias. |
|||||||||||||||
Box 5 – Randomised control trials comparing the effectiveness of faecal microbiota transplantation for curing Clostridium difficile-associated diarrhoea with treatment with vancomycin or placebo
Box 6 – Subgroup analyses of randomised control trials of faecal microbiota transplantation for treating Clostridium difficile-associated diarrhoea
|
Parameter |
Number of studies |
Number of patients |
Rate ratio (95% CI) |
I2 |
P (interaction) |
||||||||||
|
|
|||||||||||||||
|
Risk of bias |
|
|
|
|
0.53 |
||||||||||
|
Low |
3 |
111 |
0.32 (0.11–0.91) |
69% |
|
||||||||||
|
Unclear/high |
2 |
173 |
0.50 (0.20–1.26) |
49% |
|
||||||||||
|
Type of control |
|
|
|
|
0.53 |
||||||||||
|
Vancomycin |
3 |
111 |
0.32 (0.11–0.91) |
69% |
|
||||||||||
|
Autologous/placebo |
2 |
173 |
0.50 (0.20–1.26) |
49% |
|
||||||||||
|
Continent |
|
|
|
|
0.01 |
||||||||||
|
Europe |
2 |
81 |
0.20 (0.09–0.46) |
0% |
|
||||||||||
|
North America |
3 |
203 |
0.63 (0.45–0.88) |
0% |
|
||||||||||
|
Route of administration |
|
|
|
|
0.02 |
||||||||||
|
Naso-duodenal |
1 |
42 |
0.26 (0.09–0.73) |
0% |
|
||||||||||
|
Enema |
2 |
157 |
0.67 (0.47–0.94) |
0% |
|
||||||||||
|
Colonoscopy |
2 |
85 |
0.18 (0.07–0.47) |
0% |
|
||||||||||
|
|
|||||||||||||||
|
|
|||||||||||||||
Received 27 March 2017, accepted 28 June 2017
- Paul Moayyedi1
- Yuhong Yuan1
- Harith Baharith1
- Alexander C Ford2,3
- 1 McMaster University, Hamilton, ON, Canada
- 2 Leeds Gastroenterology Institute, St James's University Hospital, Leeds, United Kingdom
- 3 Institute of Biomedical and Clinical Sciences, University of Leeds, Leeds, United Kingdom
No relevant disclosures.
- 1. Hall I, O’Toole E. Intestinal flora in newborn infants with a description of a new pathogenic anaerobe, Bacillus difficilis. Am J Dis Child 1935; 49: 390.
- 2. Heinlen L, Ballard JD. Clostridium difficile infection. Am J Med Sci 2010; 340: 247-252.
- 3. Bartlett JG. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994; 18 (Suppl 4): S265-S272.
- 4. Reveles KR, Lee GC, Boyd NK, Frei CR. The rise in Clostridium difficile infection incidence among hospitalized adults in the United States: 2001–2010. Am J Infect Control 2014; 42: 1028-1032.
- 5. Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015; 372: 1539-1548.
- 6. Slimings C, Armstrong P, Beckingham WD, et al. Increasing incidence of Clostridium difficile infection, Australia, 2011–2012. Med J Aust 2014; 200: 272-276. <MJA full text>
- 7. O'Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136: 1913-1924.
- 8. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile in the United States. N Engl J Med 2015; 372: 825-834.
- 9. Zhang F, Luo W, Shi Y, et al. Should we standardize the 1700-year old fecal microbiota transplantation? Am J Gastroenterol 2012; 107: 1755.
- 10. Borody TJ, Brandt LJ, Paramsothy S. Therapeutic faecal microbiota transplantation: current status and future developments. Curr Opin Gastroenterol 2014; 30: 97-105.
- 11. Debast SB, Bauer MP, Kuipers EJ. European Society of Clinical Microbiology and Infectious Diseases: update of the treatment guidance document for Clostridium difficile infection. Clin Microbial Infect 2014; 20 (Suppl 2): 1-26.
- 12. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017; 66: 569-580.
- 13. Moayyedi P, Marshall JK, Yuan Y, Hunt R. Canadian Association of Gastroenterology position statement: fecal microbiota transplant therapy. Can J Gastroenterol Hepatol 2014; 28: 66-68.
- 14. Cheng AC, Ferguson JK, Richards MJ, et al. Australasian Society for Infectious Diseases guidelines for the diagnosis and treatment of Clostridium difficile infection. Med J Aust 2011; 194: 353-358. <MJA full text>
- 15. Drekonja D, Reich J, Gezahegn S, et al. Fecal microbiota transplantation for Clostridium difficile infection. a systematic review. Ann Intern Med 2015; 162: 630-638.
- 16. Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors). Cochrane handbook for systematic reviews of interventions; version 5.2 (updated Feb 2017), Cochrane, 2017. http://training.cochrane.org/ (accessed June 2017).
- 17. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177-188.
- 18. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557-560.
- 19. Moayyedi P. Meta-analysis: can we mix apples and oranges? Am J Gastroenterol 2004; 99: 2297-2301.
- 20. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002.
- 21. Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629-634.
- 22. van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368: 407-415.
- 23. Cammarota G, Masucci L, Ianiro G, et al. Randomised clinical trial: faecal microbiota transplantation by colonoscopy vs. vancomycin for the treatment of recurrent Clostridium difficile infection. Aliment Pharmacol Ther 2015; 41: 835-843.
- 24. Kelly CR, Khoruts A, Staley C, et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection. Ann Intern Med 2016; 165: 609-616.
- 25. Orenstein R, Dubberke E, Lee CH, et al. RBX2660, a microbiota-based drug for the prevention of recurrent Clostridium difficile infection, is safe and effective: results from a randomised, double-blinded, placebo-controlled trial (abstract LB08). 24th UEG Week 2016; Vienna (Austria), 17–19 Oct 2016. United European Gastroenterol J 2016; 4: 802.
- 26. Hota SS, Sales V, Tomlinson G, et al. Oral vancomycin followed by fecal transplantation versus tapering oral vancomycin treatment for recurrent Clostridium difficile infection: an open-label, randomized controlled trial. Clin Infect Dis 2017; 64: 265-271.
- 27. Youngster I, Sauk J, Pindar C, et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin Infect Dis 2014; 58: 1515-1522.
- 28. Allegretti JR, Fischer M, Papa E, et al. Fecal microbiota transplantation delivered via oral capsules achieves microbial engraftment similar to traditional delivery modalities: safety efficacy and engraftment results from a multi-center cluster randomized dose-finding study (abstract Su1738). Digestive Disease Week (DDW) 2016; San Diego (USA), 21–24 May 2016. Gastroenterology 2016; 150 (Suppl 1): S540.
- 29. Kao D, Roach B, Hotte N, et al. A prospective dual center, randomized trial comparing colonoscopy versus capsule delivered fecal microbiota transplantation (FMT) in the management of recurrent Clostridium difficile infection (RCDAD) (poster A117). Canadian Digestive Diseases Week 2016; Montreal (Canada), 26–29 Feb 2016. Can J Gastroenterol Hepatol 2016: article 4792898, p. 71.
- 30. Lee CH, Steiner T, Petrof EO, et al. Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection a randomized clinical trial. JAMA 2016; 315: 142-149.
- 31. Jiang ZD, Ajami NJ, Petrosino JF, et al. Randomised clinical trial: faecal microbiota transplantation for recurrent Clostridum difficile infection — fresh, or frozen, or lyophilised microbiota from a small pool of healthy donors delivered by colonoscopy. Aliment Pharmacol Ther 2017; 45: 899-908.
- 32. Guyatt GH, Oxman AD, Vist G, et al; for the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924-926.
- 33. Rossen NG, MacDonald JK, de Vries EM, et al. Fecal microbiota transplantation as novel therapy in gastroenterology: a systematic review. World J Gastroenterol 2015; 21: 5359-5371.
- 34. Chapman BC, Moore HB, Overby DM, et al. Fecal microbiota transplant in patients with Clostridium difficile infection: a systematic review. Acute Care Surg 2016; 81: 756-764.
- 35. Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 2013; 108: 500-508.
- 36. Borody TJ, George L, Andrews P, et al. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust 1989; 150: 604.
- 37. Costello SP, Conlon MA, Vuaran MS, et al. Faecal microbiota transplant for recurrent Clostridium difficile infection using long-term frozen stool is effective: clinical efficacy and bacterial viability data. Aliment Pharmacol Ther 2015; 42: 1011-1018.
- 38. Borody TJ, Fischer M, Mitchell S, Campbell J. Fecal microbiota transplantation in gastrointestinal disease: 2015 update and the road ahead. Expert Rev Gastroenterol Hepatol 2015; 9: 1379-1391.
- 39. University of Texas Health Science Center, Houston. Fecal microbiota transplantation to treat recurrent C. difficile associated diarrhea via retention enema or oral route. https://clinicaltrials.gov/ct2/show/NCT02449174 (accessed June 2017).
- 40. Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med 2011; 364: 422-431.
- 41. Baxter M, Ahmad T, Colville A, Sheridan R. Fatal aspiration pneumonia as a complication of fecal microbiota transplantation. Clin Inf Dis 2015; 61: 136-137.





Abstract
Objectives: Faecal microbiota transplantation (FMT) has emerged as a useful approach for treating Clostridium difficile-associated diarrhoea (CDAD). Randomised controlled trials (RCTs) have recently evaluated its effectiveness, but systematic reviews have focused on evidence from case series. We therefore conducted a systematic review and meta-analysis of RCTs evaluating the effectiveness of FMT for treating CDAD.
Study design: We included RCTs that primarily recruited adults with CDAD and compared the effectiveness of FMT with that of placebo, antibiotic therapy, or autologous stool transplantation, or compared different preparations or modes of delivery of FMT. Dichotomous symptom data were pooled to calculate a relative risk (RR) of CDAD persisting after therapy, and the number needed to treat (NNT).
Data sources: MEDLINE, EMBASE, and the Cochrane Controlled Trials Register and Database of Systematic Reviews were searched to 6 February 2017.
Data synthesis: We identified ten RCTs that evaluated the treatment of a total of 657 patients with CDAD. Five RCTs compared FMT with placebo (including autologous FMT) or vancomycin treatment (total of 284 patients); FMT was statistically significantly more effective (RR, 0.41; 95% CI, 0.22–0.74; NNT, 3; 95% CI, 2–7). Heterogeneity across studies was significant (I2 = 61%); this heterogeneity was attributable to the mode of delivery of FMT, and to the therapy being more successful in European than in North American trials. The other five RCTs evaluated different approaches to FMT therapy. Frozen FMT preparations were as efficacious as fresh material in one RCT, but the numbers of patients in the remaining RCTs were too small to allow definitive conclusions.
Conclusions: Moderate quality evidence from RCT trials indicates that FMT is more effective in patients with CDAD than vancomycin or placebo. Further investigations are needed to determine the best route of administration and FMT preparation.