Rotavirus gastroenteritis is the leading cause of severe acute gastroenteritis (AGE) in children aged less than 5 years; it results in over half a million deaths each year, most of which occur in developing countries.1 Most children worldwide are infected with rotavirus by the age of 5 years, with severe disease occurring most commonly between the ages of 6 months and 2 years.1
In Australia, before a rotavirus vaccination program was introduced, this virus was responsible for more than 10 000 hospitalisations of children aged under 5 years annually, placing an enormous burden on paediatric hospitals.2-4 The burden of disease in Aboriginal and Torres Strait Islander children was much greater, with a hospitalisation rate about five times that of non-Indigenous children aged less than 12 months.5 Large outbreaks in Indigenous children occur on a background of endemic infection in central and northern Australia.6 There was about one rotavirus-related death in Australia annually, affecting the very young or, rarely, elderly adults.5
Australia was one of the first countries to introduce a nationally funded rotavirus vaccination program.7 Two vaccines were registered in Australia in May 2006: Rotarix (GlaxoSmithKline Biologicals) and RotaTeq (CSL Biotherapies/Merck & Co). Both are oral live attenuated vaccines for use in infants, but there are differences in their composition and dosing. Rotarix is an attenuated human vaccine containing the G1P1[8] strain of rotavirus which is given in a two-dose course at 2 and 4 months of age, whereas RotaTeq is a human-bovine reassortant vaccine containing 5 strains (G1, G2, G3, G4, and P1[8]), which is given as three doses at 2, 4 and 6 months of age. The Northern Territory was the first jurisdiction to fund rotavirus vaccination, beginning in October 2006; from July 2007 both vaccines were included in the National Immunisation Program (NIP) for all Australian infants.7 The vaccine used varies by jurisdiction, and there are upper age limits on doses, which preclude “catch up” vaccination.7,8 Some Australian jurisdictions and paediatric hospitals have reported a decline in rotavirus disease on the basis of laboratory testing,9,10 notification data10,11 and hospitalisations12-15 for the first 1 to about 3 years of the program. In addition, early estimates of vaccine effectiveness suggest that although vaccination provides a high level of protection against hospitalisation at a population level (in Queensland),14 its effectiveness in disease outbreaks in Indigenous children in central Australia is variable.16,17
This study is the first to examine national data on all hospitalisations for AGE. Our aim was to assess the impact of vaccination on hospitalisation rates, focusing on differences related to age, Indigenous status and location.
Hospitalisation data were obtained from the National Hospital Morbidity Database of the Australian Institute of Health and Welfare (AIHW). This database is an electronic collection of de-identified records of patients admitted to public and private hospitals in Australia. Hospitalisations were analysed if they had separation dates between 1 July 2001 and 30 June 2010 with either a principal or additional diagnosis that had International Classification of Diseases, 10th Revision, Australian Modification (ICD-10-AM) codes for rotavirus enteritis (A08.0) or any other AGE not coded as rotavirus (K52 and A01 to A09, excluding A08.0).
To enable comparison by Indigenous status, data were used only for jurisdictions and periods for which Indigenous identification was considered acceptable by the AIHW.18 Thus, data from 2004 onwards for New South Wales, Victoria, Queensland, Western Australia and South Australia, and from 2001 for the NT, were analysed.
We calculated hospitalisation rates using annual population estimates from the Australian Bureau of Statistics Australian demographic statistics.19 Age-specific rates, incidence rate ratios (IRRs) and 95% confidence intervals were calculated.
We assessed rotavirus vaccine coverage using data from the Australian Childhood Immunisation Register (ACIR), a national register administered by Medicare Australia that records details of vaccinations given to children aged under 7 years.20
We performed all analyses using SAS statistical software, version 9.2 (SAS Institute Inc).
From July 2001 to June 2010, there were 28 502 hospitalisations coded as rotavirus gastroenteritis. Box 1 shows that the annual winter/spring peak in rotavirus-coded hospitalisations was reduced after vaccination was introduced in July 2007, after which there was further substantial attenuation in the peak for the next two seasons. This decline in disease coincided with the rapid attainment and maintenance of a national vaccine coverage level of about 85% for a full vaccine course (either two or three doses) by 12 months of age (Box 1).
As seen in Box 2, rates were highest among children 1 year of age before rotavirus vaccination was introduced, with the greatest declines in rates occurring in this group and the 2-year-old age group (86% and 70%, respectively) by 2009–10 (Box 2 and Box 3). Overall, for children aged less than 5 years, there was a 71% decline in hospitalisations between periods before and after rotavirus vaccination (from 261 per 100 000 to 75 per 100 000). Rates also decreased after vaccination compared with the prevaccination years, for all other groups aged less than 20 years. However, there were small increases in rotavirus-coded hospitalisation rates among adult age groups in the years after rotavirus vaccination was introduced (Box 2).
There were 1 207 978 hospitalisations for AGE not coded as rotavirus gastroenteritis from July 2001 to June 2010. About 82% were assigned one or both of the two most common ICD-10-AM codes — A09 (other gastroenteritis and colitis of infectious and unspecified origin) and K52 (other non-infective gastroenteritis and colitis). AGE hospitalisation rates were highest in children aged less than 1 year, among whom there was a 23% rate reduction in 2009-10 compared to the period before rotavirus vaccination was introduced. Among children aged less than 5 years, there was a 38% decline in hospitalisations (from 1419 to 880 per 100 000). As with rotavirus-coded hospitalisations, rates decreased in the postvaccination periods for all age groups aged less than 20 years, but increased in older adults, although the absolute rates remained low (Box 2).
There were differences in rates and rate reductions across states and territories. Declines in 2009–10 compared with the prevaccination period ranged from 34% in the NT to 83% in Tasmania (data not shown). In the NT, there was an overall downward trend in rates of hospitalisations for both rotavirus and other AGE after the vaccination program was introduced in late 2006. However, there were increases in rates of hospitalisation for rotavirus gastroenteritis in 2008–09 and 2009–10 (Box 4).
There was an overall decline in AGE (rotavirus- and non-rotavirus-coded) hospitalisation rates in Indigenous children (Box 5), although declines were smaller than those in non-Indigenous children. There was a marked decline in AGE hospitalisation rates in Indigenous children aged less than 1 year, particularly in those coded as rotavirus gastroenteritis, which declined by 29% from 1465 to 1045 per 100 000 in 2009–10 (Box 5). However, no decline was observed in the 5–19-years age group; this is in contrast to the significant reduction in hospitalisations for rotavirus gastroenteritis among non-Indigenous children of this age.
Box 4 shows rotavirus-coded hospitalisation rates in Indigenous and non-Indigenous children aged under 5 years in the NT compared with NSW, Victoria, Queensland, SA and WA combined. Higher rates were observed in Indigenous children than non-Indigenous children; this was most marked in the NT, but also occurred in the other states, and persisted after the introduction of the rotavirus vaccination program. In the postvaccination period, hospitalisations remained lower in non-NT resident Indigenous children, but there were annual fluctuations in hospitalisation rates in the NT.
This study is the first to present national data showing substantial declines in both rotavirus-coded and non-rotavirus-coded hospitalisations for AGE since the introduction of rotavirus vaccines to the Australian NIP. Declines in numbers of hospitalisations were evident immediately from when the program commenced in 2007, consistent with the rapid attainment of high levels of vaccine coverage.21 As expected, the greatest impact has been in children aged less than 3 years, who were eligible to receive the vaccine during the study period. Our results are similar to those of studies from SA, Queensland, and NSW.13-15 The decline in non-rotavirus-coded AGE hospitalisations is not unexpected given that estimates of rotavirus-related hospitalisations before rotavirus vaccines were available exceeded those derived using rotavirus-specific coding,4,5 and NSW data indicate that the sensitivity of the rotavirus discharge code was only 48%, although its specificity was about 99% and the positive predictive value was 88% (S Jayasinghe, Senior Policy Officer, National Centre for Immunisation Research and Surveillance [NCIRS], personal communication). However, an impact on hospitalisation or emergency department care for all-cause AGE was also seen in trials of the two available rotavirus vaccines, in which testing for rotavirus was more rigorous.22,23
Nationally, there were significant declines in hospitalisations for rotavirus and other forms of AGE in those aged between 3 and 19 years after the introduction of the rotavirus vaccination program. This suggests a herd immunity effect, as only children aged less than 3 years were eligible to receive rotavirus vaccine during the study period. A decline in hospitalisations for AGE has also been observed in earlier studies in Australia13-15 and the United States,24 and this should be factored in to any cost-effectiveness assessments of the rotavirus vaccination program.
We found modest but significant increases in national hospitalisation rates for both rotavirus and non-rotavirus AGE in adults, particularly those aged 65 years or older. This increase occurred gradually across the whole study period, although absolute rates, particularly for rotavirus-coded gastroenteritis, remained low. This finding is consistent with the observation that rotavirus has been increasingly recognised as a cause of morbidity in older patients.25 Similar changes have been reported in Queensland17,18 and may relate to increased testing for rotavirus and other agents of gastroenteritis, such as adenovirus and norovirus, in older people.10,14
Before rotavirus vaccination was introduced, Indigenous children, especially in central Australia, had the greatest burden of rotavirus disease.5,6 Our study identified that, across Australia, hospitalisation rates for Indigenous children have declined in the postvaccination period. However, the reduction has been smaller than for non-Indigenous children. Further, in the NT, where hospitalisation rates for rotavirus and AGE in Indigenous children were markedly higher before rotavirus vaccination, the reduction in disease has been less substantial and inconsistent. For example, hospitalisations rose in the NT, in 2008 and 2009, associated with an outbreak of the G2P[4] rotavirus strain,11 suggesting that heterotypic immunity from the monovalent G1P[8] strain vaccine (Rotarix) was reduced in that population.16 The effectiveness of the vaccine in an earlier NT outbreak of the G9P[8] rotavirus strain was higher (84.5%; 95% CI, 23.4%–96.9%);17 however, this same strain was circulating in the 2009–10 financial year, when hospitalisation rates increased again. Overall, vaccination has had less impact on rotavirus in this setting of high disease burden and a vulnerable population than in Indigenous and non-Indigenous children elsewhere in Australia. Although vaccine coverage is about 16% lower in Indigenous than in non-Indigenous children (71.5% and 84.5% by age 12 months, respectively),21 this alone does not appear to explain the reduced impact of the vaccine, which is more in keeping with that seen in efficacy studies in developing countries.26
Our study had several limitations. Its design was descriptive and ecological, and outcomes may reflect factors not related to immunisation, such as strain variation and coding practices. However, natural fluctuations in rotavirus disease activity are known to occur, often related to the emergence of new strains; this emphasises the importance of assessing vaccine impact over many rotavirus seasons.7,15 In addition, although hospitalisation databases may directly reflect the rotavirus disease burden, data suggest that the positive predictive value and specificity of coding for rotavirus has not changed since rotavirus vaccination began, and together with non-rotavirus coded hospitalisation data, remains useful for assessing trends in hospitalisation rates (S Jayasinghe, Senior Policy Officer, NCIRS, personal communication). As vaccination status was not available from the de-identified data used in this study, vaccine effectiveness could not be determined. More studies are required to compare the long-term effectiveness of vaccination in Indigenous children in differing regions of Australia, and to assess the changing epidemiology of acute gastroenteritis across all age groups.
1 Number of hospitalisations* coded as rotavirus gastroenteritis (all ages) and infant rotavirus vaccine coverage,† Australia, July 2001 to June 2010
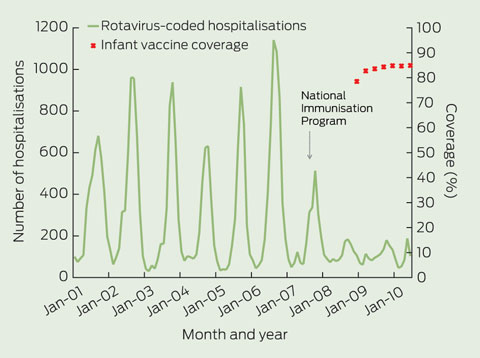
* By month of admission. † Coverage was calculated using data from the Australian Childhood Immunisation Register by 3-month birth cohorts born between 1 July 2007 and 31 March 2009, and the coverage assessment date was 12 months after the last birth date of each cohort.
2 Hospitalisation rates (per 100 000 population) for acute gastroenteritis (rotavirus- and non-rotavirus-coded), by age group, before and after the introduction of rotavirus vaccine, Australia, July 2001 to June 2010*
|
Before vaccination |
After vaccination |
|||||||||||||
|
2001–02 to 2005–06 |
Year 1 2007–08 |
Year 2 2008–09 |
Year 3 2009–10 |
|||||||||||
Patient age (years) |
Mean annual rate |
Rate |
IRR† (95% CI) |
Rate |
IRR† (95% CI) |
Rate |
IRR† (95% CI) |
||||||||
Rotavirus-coded hospitalisations |
|
|
|
|
|
|
|
|
|||||||
< 1 |
387.7 |
195.3 |
0.50 (0.46–0.55) |
99.8 |
0.26 (0.23–0.29) |
135.3 |
0.35 (0.31–0.39) |
||||||||
1 |
483.9 |
300.1 |
0.62 (0.58–0.67) |
110.2 |
0.23 (0.20–0.25) |
66.59 |
0.14 (0.12–0.16) |
||||||||
2 |
250.9 |
134.3 |
0.54 (0.48–0.60) |
102.3 |
0.41 (0.36–0.46) |
74.8 |
0.30 (0.26–0.34) |
||||||||
3 |
122.4 |
54.5 |
0.45 (0.38–0.53) |
40.9 |
0.33 (0.28–0.40) |
65.1 |
0.53 (0.46–0.62) |
||||||||
4 |
67.4 |
37.3 |
0.55 (0.45–0.68) |
23.6 |
0.35 (0.27–0.45) |
32.2 |
0.48 (0.39–0.59) |
||||||||
5–19 |
6.7 |
3.5 |
0.52 (0.44–0.61) |
2.9 |
0.43 (0.36–0.52) |
3.4 |
0.51 (0.43–0.60) |
||||||||
20–44 |
0.2 |
0.4 |
1.54 (1.00–2.37) |
0.4 |
1.67 (1.10–2.54) |
0.5 |
1.96 (1.32–2.89) |
||||||||
45–64 |
0.4 |
0.6 |
1.54 (1.02–2.33) |
0.5 |
1.51 (1.00–2.29) |
0.6 |
1.63 (1.10–2.44) |
||||||||
≥ 65 |
1.6 |
4.4 |
2.73 (2.19–3.41) |
3.3 |
2.04 (1.60–2.59) |
3.6 |
2.24 (1.78–2.83) |
||||||||
Acute gastroenteritis hospitalisations (excluding those coded as rotavirus) |
|||||||||||||||
< 1 |
2094.1 |
2010.7 |
0.96 (0.93–0.99) |
1581.3 |
0.76 (0.73–0.78) |
1607.9 |
0.77 (0.74–0.79) |
||||||||
1 |
2233.1 |
1874.2 |
0.84 (0.81–0.86) |
1334.8 |
0.6 (0.58–0.62) |
1146.5 |
0.51 (0.50–0.53) |
||||||||
2 |
1337.4 |
935.2 |
0.70 (0.67–0.73) |
823.8 |
0.62 (0.59–0.64) |
721.8 |
0.54 (0.52–0.56) |
||||||||
3 |
849.4 |
643.9 |
0.76 (0.72–0.80) |
507.6 |
0.60 (0.57–0.63) |
536.3 |
0.63 (0.60–0.67) |
||||||||
4 |
609.4 |
478.2 |
0.78 (0.74–0.83) |
358.8 |
0.59 (0.55–0.63) |
365.6 |
0.60 (0.56–0.64) |
||||||||
5–19 |
246.1 |
246.3 |
1.00 (0.98–1.02) |
231.9 |
0.94 (0.92–0.96) |
227.7 |
0.92 (0.91–0.95) |
||||||||
20–44 |
408.0 |
465.7 |
1.14 (1.13–1.15) |
455.8 |
1.12 (1.10–1.13) |
472.8 |
1.16 (1.15–1.17) |
||||||||
45–64 |
492.6 |
588.9 |
1.20 (1.18–1.21) |
592.8 |
1.20 (1.19–1.22) |
638.5 |
1.30 (1.28–1.31) |
||||||||
≥ 65 |
1496.0 |
1974.1 |
1.32 (1.31–1.33) |
1862.9 |
1.25 (1.23–1.26) |
2018.3 |
1.35 (1.34–1.36) |
||||||||
IRR = incidence rate ratio. * Hospitalisations by separation dates within financial years: a mean annual rate was calculated for July 2001 to June 2006. † Comparison of each year after vaccination with the before-vaccination period (July 2001 to June 2006). |
|||||||||||||||
3 Comparison of rotavirus-coded hospitalisation rates by age in children aged < 5 years, before and for 3 years after the introduction of rotavirus vaccine, Australia, July 2001 to June 2010*
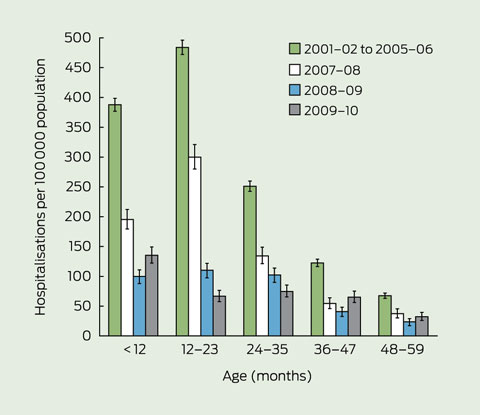
* Rotavirus hospitalisations: ICD-10-AM code for rotavirus enteritis (A08.0) in any field (principal diagnosis and additional diagnosis) was used; a mean annual rate was calculated for July 2001 to June 2006.
4 Rotavirus-coded hospitalisation rates in Indigenous children aged < 5 years in the Northern Territory, compared with five other Australian jurisdictions combined, July 2001 to June 2010*
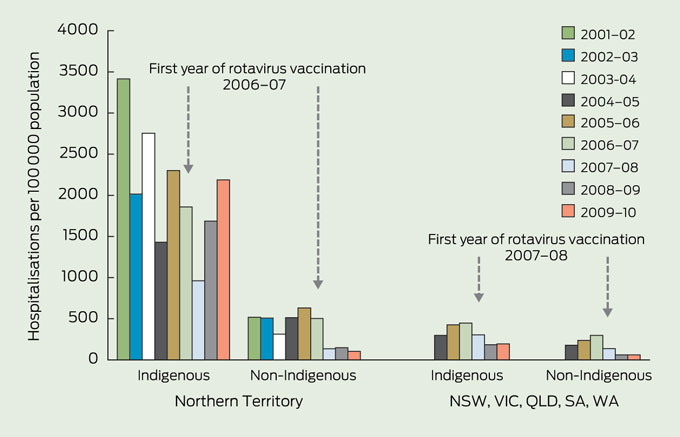
* The jurisdictions with acceptable level of > 80% Indigenous identification were the Northern Territory, New South Wales, Queensland, South Australia, Victoria and Western Australia.18
5 Hospitalisation rates (per 100 000 population) for Indigenous people for acute gastroenteritis (rotavirus- and non-rotavirus-coded), by age group, before and after the introduction of rotavirus vaccine, Australia,* July 2004 to June 2010
|
Before vaccination |
After vaccination |
|||||||||||||
|
2004–05 to 2005–06† |
Year 1 2007–08† |
Year 2 2008–09† |
Year 3 2009–10† |
|||||||||||
Patient age (years) |
Mean annual rate |
Rate |
IRR‡ (95% CI) |
Rate |
IRR‡ (95% CI) |
Rate |
IRR‡ (95% CI) |
|
|||||||
Rotavirus-coded hospitalisations* |
|
|
|
|
|
||||||||||
< 1 |
1465 |
904 |
0.62 (0.50–0.76) |
825 |
0.56 (0.45–0.70) |
1045 |
0.71 (0.58–0.87) |
|
|||||||
1–4 |
280 |
245 |
0.87 (0.70–1.09) |
233 |
0.83 (0.67–1.04) |
257 |
0.92 (0.74–1.14) |
|
|||||||
5–19 |
2 |
3 |
1.4 (0.44–4.42) |
3 |
1.67 (0.56–4.98) |
3 |
1.39 (0.44–4.39) |
|
|||||||
20–44 |
1 |
0 |
— |
1 |
1.24 (0.21–7.40) |
1 |
0.62 (0.06–5.94) |
|
|||||||
45–64 |
1 |
1 |
1.82 (0.11–29.04) |
1 |
1.75 (0.11–28.03) |
6 |
7.01 (0.78–62.74) |
|
|||||||
≥ 65 |
— |
— |
|
— |
|
— |
|
|
|||||||
Acute gastroenteritis hospitalisations (excluding those coded as rotavirus)* |
|
|
|
||||||||||||
< 1 |
7377 |
6850 |
0.93 (0.86–1.01) |
4943 |
0.67 (0.61–0.73) |
5761 |
0.78 (0.72–0.85) |
|
|||||||
1–4 |
2947 |
2715 |
0.92 (0.86–0.98) |
2222 |
0.75 (0.70–0.81) |
2134 |
0.72 (0.67–0.78) |
|
|||||||
5–19 |
281 |
404 |
1.44 (1.30–1.58) |
255 |
0.91 (0.81–1.02) |
255 |
0.91 (0.81–1.02) |
|
|||||||
20–44 |
524 |
615 |
1.18 (1.09–1.27) |
549 |
1.05 (0.97–1.13) |
529 |
1.01 (0.94–1.09) |
|
|||||||
45–64 |
900 |
1163 |
1.29 (1.18–1.42) |
1096 |
1.22 (1.11–1.34) |
1318 |
1.46 (1.34–1.60) |
|
|||||||
≥ 65 |
2158 |
3353 |
1.55 (1.38–1.76) |
2711 |
1.26 (1.10–1.43) |
3034 |
1.41 (1.24–1.59) |
|
|||||||
IRR = incidence rate ratio. * Northern Territory, New South Wales, Queensland, South Australia, Victoria and Western Australia with acceptable level of ≥ 80% Indigenous identification. † Hospitalisations by separation dates within financial years. ‡ Comparison of each postvaccination year with the prevaccination period (July 2004 to June 2006). |
|
||||||||||||||
Received 12 January 2012, accepted 22 July 2012
- Aditi Dey1,2
- Han Wang1
- Robert Menzies1,2
- Kristine Macartney1,2
- 1 National Centre for Immunisation Research & Surveillance (NCIRS), Sydney, NSW.
- 2 Discipline of Paediatrics and Child Health, University of Sydney, Sydney, NSW.
The NCIRS is supported by the Australian Government Department of Health and Ageing, the NSW Department of Health and the Children’s Hospital at Westmead. We thank Donna Armstrong of the NCIRS for her constructive feedback during the preparation of this manuscript.
No relevant disclosures.
- 1. Parashar UD, Alexander JP, Glass RI. Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm and Rep 2006; 55: 1-13.
- 2. Carlin JB, Chondros P, Masendycz P, et al. Rotavirus infection and rates of hospitalisation for acute gastroenteritis in young children in Australia, 1993-1996. Med J Aust 1998; 169: 252-256. <MJA full text>
- 3. Chiu C, Dey A, Wang H, et al. Vaccine preventable diseases in Australia, 2005 to 2007. Commun Dis Intell 2010; 34 Suppl: S1-S167.
- 4. Galati JC, Harsley S, Richmond P, Carlin JB. The burden of rotavirus-related illness among young children on the Australian health care system. Aust N Z J Public Health 2006; 30: 416-421.
- 5. Newall AT, MacIntyre CR, Wang H, et al. Burden of severe rotavirus disease in Australia. J Paediatr Child Health 2006; 42: 521-527.
- 6. Schultz R. Rotavirus gastroenteritis in the Northern Territory, 1995-2004. Med J Aust 2006; 185: 354-356. <MJA full text>
- 7. Macartney KK, Burgess MA. Rapid impact of rotavirus vaccination in the United States: implications for Australia. Med J Aust 2009; 191: 131-132. <MJA full text>
- 8. National Health and Medical Research Council. Australian immunisation handbook. 9th ed. Canberra: NHMRC, 2008.
- 9. Belshaw DA, Muscatello DJ, Ferson MJ, Nurkik A. Rotavirus vaccination one year on. Commun Dis Intell 2009; 33: 337-340.
- 10. Lambert SB, Faux CE, Hall L, et al. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med J Aust 2009; 191: 157-160.
- 11. Cook H, Krause V. A review of G2P[4] rotavirus outbreaks in Central Australia and how the introduction of Rotarix has affected the epidemiology. The Northern Territory Disease Control Bulletin 2010; 17: 14-21.
- 12. Buttery JP, Lambert SB, Grimwood K, et al. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia’s national childhood vaccine schedule. Pediatr Infect Dis J 2011; 30: S25-S29.
- 13. Clarke MF, Davidson GP, Gold MS, Marshall HS. Direct and indirect impact on rotavirus positive and all-cause gastroenteritis hospitalisations in South Australian children following the introduction of rotavirus vaccination. Vaccine 2011; 29: 4663-4667.
- 14. Field EJ, Vally H, Grimwood K, Lambert SB. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics 2010; 126: e506-e512.
- 15. Macartney KK, Porwal M, Dalton D, et al. Decline in rotavirus hospitalisations following introduction of Australia’s national rotavirus immunisation program. J Paediatr Child Health 2011; 47: 266-270.
- 16. Snelling TL, Andrews RM, Kirkwood CD, et al. Case-control evaluation of the effectiveness of the G1P[8] human rotavirus vaccine during an outbreak of rotavirus G2P[4] infection in Central Australia. Clin Infect Dis 2011; 52: 191-199.
- 17. Snelling TL, Schultz R, Graham J, et al. Rotavirus and the Indigenous children of the Australian outback: monovalent vaccine effective in a high-burden setting. Clin Infect Dis 2009; 49: 428-431.
- 18. Australian Institute of Health and Welfare. Indigenous identification in hospital separations data-quality report. Canberra: AIHW, 2010. (AIHW Cat. No. HSE 85; Health Services Series No. 35.)
- 19. Australian Bureau of Statistics. Australian Demographic Statistics. (ABS Cat. No. 3101.0.) http://www.abs.gov.au/ausstats/abs@.nsf/mf/3101.0/ (accessed Aug 2012).
- 20. Medicare Australia. Australian Childhood Immunisation Register. 2012. http://www. medicareaustralia.gov.au/public/services/acir/index.jsp (accessed Mar 2012).
- 21. Hull B, Dey A, Mahajan D, et al. Immunisation coverage annual report, 2009. Commun Dis Intell 2011; 35: 132-148.
- 22. Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354: 11-22.
- 23. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354: 23-33.
- 24. Lopman BA, Curns AT, Yen C, Parashar UD. Infant rotavirus vaccination may provide indirect protection to older children and adults in the United States. J Infect Dis 2011; 204: 980-986.
- 25. Anderson EJ, Weber SG. Rotavirus infection in adults. Lancet Infect Dis 2004; 4: 91-99.
- 26. Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med 2010; 362: 289-298.





Abstract
Objective: To evaluate the impact of the Australian rotavirus vaccination program on both rotavirus and all-cause acute gastroenteritis (AGE) hospitalisations and to compare outcomes in Indigenous and non-Indigenous people.
Design and setting: Retrospective analysis of the Australian Institute of Health and Welfare National Hospital Morbidity database for hospitalisations coded as rotavirus and all-cause AGE, between 1 July 2001 and 30 June 2010.
Main outcome measures: Age-specific hospitalisation rates in Indigenous and non-Indigenous people, before and after the introduction of the vaccine program in July 2007.
Results: There was a 71% decline in rotavirus-coded hospitalisations of children aged < 5 years between periods before and after rotavirus vaccination (from 261 per 100 000 to 75 per 100 000). There was also a 38% decline in non-rotavirus coded AGE hospitalisations (from 1419 per 100 000 to 880 per 100 000). This represented more than 7700 hospitalisations of children aged < 5 years being averted in the financial year 2009–10. Reductions were also observed in the 5–19-years age group, suggesting that transmission of virus was reduced at a population level. Decreases in hospitalisations of Indigenous children were smaller than those for the general population, and fluctuated by location and year.
Conclusions: These data show a sustained and substantial decline in severe rotavirus disease and all-cause AGE since the introduction of rotavirus vaccination, most pronounced in the target age group, but with evidence of herd immunity. The impact of rotavirus vaccination in Indigenous children in hyperendemic settings was less remarkable.