Asthma is a major contributor to the burden of illness in children.1 In addition to specific medical treatments, parents and carers need information and skills to facilitate effective self-management2 and manage asthma exacerbations in children.3 Consistent with this, written asthma action plans (WAAPs), patient education and regular review are central components of paediatric asthma management.4
However, there is a gap between best practice and clinical care. Fewer than 25% of people with asthma have a WAAP.5 In Australia, this gap is evident in primary care, where most children with asthma are managed.1,6-9 The objective of this study was to evaluate the impact of the Practitioner Asthma Communication and Education (PACE) Australia program — an innovative communication and paediatric asthma management program for general practitioners — on asthma management and patient outcomes.
The PACE Australia program is made up of two structured, 3-hour, interactive small-group workshops (up to 10 GPs per workshop), held 1 week apart. The workshops were adapted for Australia from the original PACE program in the United States.10 A respiratory paediatrician and community physician led the topic discussions. A GP presenter discussed the Asthma Cycle of Care, which reimburses GPs for two asthma-related consultations within 12 months.11 The content of the workshops was based on five themes: assessment of the pattern of asthma; appropriate use of medications; provision of a WAAP; doctor–patient communication; and patient education. A video demonstrating 10 communication12 and asthma education strategies was shown.
Outcome data for the GPs and patients were assessed via the questionnaires used in the PACE USA trial,13 which measured communication and asthma management behaviour. GPs completed a self-administered questionnaire and the parents and carers of patients completed a telephone questionnaire (administered by an external research organisation) at baseline and 12 months after intervention or, if in the control group, 12 months after enrolment.
The study was powered for the proportion of patients provided with a WAAP. We estimated an event rate, at 12-months, of 40% for the control group14 and 70% for the intervention group. To provide adequate statistical power (power, 80%; P < 0.05) to demonstrate a between-groups difference of 30% in the primary outcome at 12 months, we set out to enrol 60 GPs per group, expecting that 45 per group would complete the study. With an average of four patients per GP, we estimated that this would provide a sample size of 240 patients per group and that 180 patients per group would complete the study.
Data were analysed using the intention-to-treat principle, with GP data analysed in the group to which the GP was allocated and patient data analysed in the group to which the patient’s GP was allocated. For GP communication style and teaching behaviour, 10 items for confidence, helpfulness and frequency domains were each summed; GPs who had a higher score at 12 months than at baseline were considered to have improved their communication skills. The impact of the intervention at 12 months was assessed using continuity-corrected χ2 tests. To adjust outcomes at 12 months for baseline values, logistic regression was used for binary variables and linear regression for continuous variables. For binary variables with a significant difference between groups, number needed to treat (NNT) was calculated — for example, the number of GPs who need to receive training for one GP to change their communication style or for one patient to receive a change in asthma care. We also adjusted for clustering of GPs by practice and patients by GP. Within-cluster intra-class correlation coefficients were computed and used to adjust Wald and t values.15 Analyses were performed using SPSS version 15.0 (SPSS Inc, Chicago, Ill, USA).
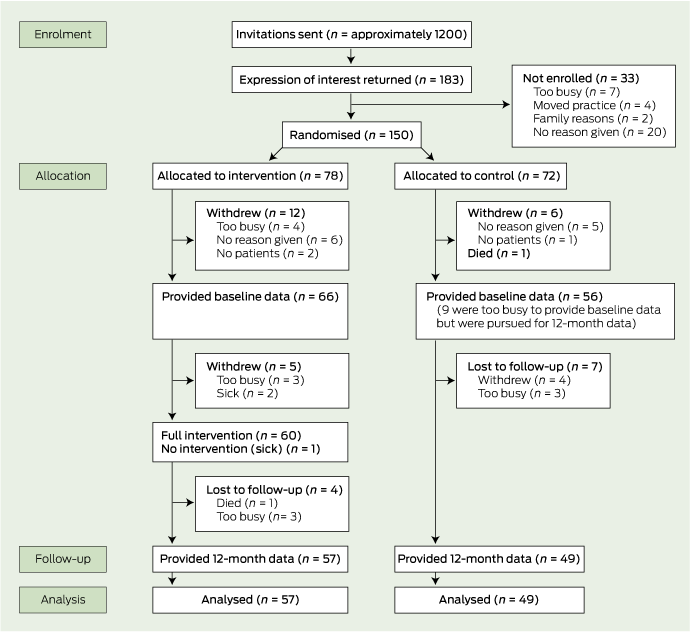
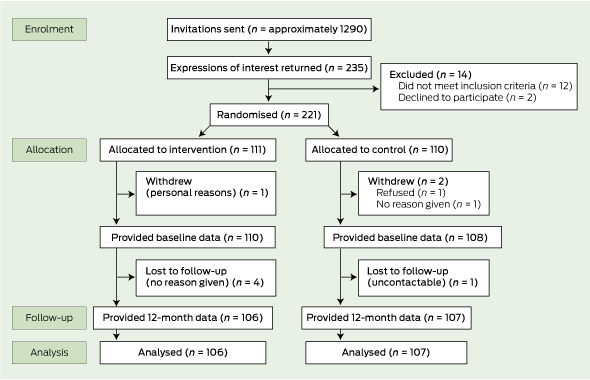
We recruited 150 GPs from 109 practices. In the intervention group, 66 GPs provided baseline data, 60 participated in the workshops and 57 provided 12-month follow-up data. In the control group, 56 provided baseline data and 49 provided 12-month follow-up data (Box 1). A total of 221 patients were enrolled; baseline data were provided for 218 patients, 12-month follow-up data were provided for 213 patients (96%) (Box 2). One hundred and sixty-six patients (75%) attended the same GP throughout the study The patients who no longer saw the same GP were included in the analysis and attended other GPs for asthma care.
GP and family characteristics were mostly balanced across the control and intervention groups (Box 3). More GPs in the control group graduated overseas, but this characteristic did not have a significant univariate association with any of the outcome variables (P > 0.1 for all associations). For families, there were differences in language other than English spoken at home and person in household who smokes, but these variables were not found to be significant univariate predictors of the primary outcomes (P > 0.1 for all associations). Asthma symptoms (as defined in the national asthma guidelines16) were infrequent intermittent for 45% of children, frequent intermittent for 46%, and persistent for 10%.
The self-reported GP outcomes (Box 4) — which reflect the national asthma guidelines16 and PACE Australia objectives — show that the frequency of providing a WAAP increased in both groups over the study period, more so in the intervention group. At 12 months, there was a 23% between-group difference in self-reported provision of a WAAP more than 70% of the time. GPs in the intervention group also had a higher rate of asking the parent or carer to demonstrate how they would use the asthma device and a higher rate of prescribing spacer devices more than 90% of the time. Data on use of the Asthma Cycle of Care was not collected at baseline, but there was a 29% higher rate of increased use of this item in the intervention group compared with the control group at 12 months, which was statistically significant (P < 0.001); this translates to three GPs needing to attend the training workshops for one extra child to complete the Asthma Cycle of Care.
In the intervention group, 15% more children received a WAAP than in the control group at 12 months, a statistically significant difference (P = 0.046 after adjusting for clustering) (Box 5). Between-group differences in asthma-related parent days away from work and child days away from school or child care were not statistically significant at 12 months, although the proportions of 1 or more days of each were lower in the intervention group. There was no significant difference in frequency of hospital visits for urgent asthma care between groups at 12 months. The frequency of discussing patient fears “very often or always” increased by 20% in the intervention group and 26% in the control group (P = 0.56).
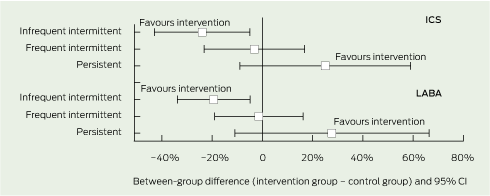
Favourable changes in family-reported use of inhaled corticosteroids and long-acting β-agonists were associated with the intervention at 12 months (Box 6). Children in the intervention group who had infrequent intermittent asthma symptoms had lower use of inhaled corticosteroids (difference, 24%; 95% CI, − 43% to − 5%; P = 0.03) and long-acting β-agonists (difference, 19%; 95% CI, − 34% to − 5%; P = 0.02). Conversely, children in the intervention group who had persistent asthma symptoms had higher use of inhaled corticosteroids (difference, 25%; 95% CI, − 9% to 59%; P = 0.4) and long-acting β-agonists (difference, 28%; 95% CI, − 11% to 66%; P = 0.4), although the confidence intervals were wide and the differences not statistically significant. In children who had frequent intermittent asthma symptoms, there was little difference between groups in the use of inhaled corticosteroids (difference, 3%; 95% CI, − 23% to 17%; P = 0.9) or long-acting β-agonists (difference, − 1%; 95% CI, − 19% to 16%; P = 1.0).
Significantly more children received WAAPs, as reported by GPs as well as parents and carers, in the intervention arm of our study. Prescribed medications also more closely reflected the child’s clinical pattern of asthma (in line with asthma guidelines16) and there was greater prescription of spacer devices. The intervention also resulted in significant improvements in GPs’ self-reported confidence when communicating with patients. The NNT values for many of the clinically important outcomes were low — ranging from 3 to 9 — suggesting that the PACE Australia program is both effective and efficient.
The PACE workshops aimed to change GP behaviour and specifically focused on improving communication skills. Appropriate prescription of inhaled corticosteroids and long-acting β-agonists according to the pattern of asthma was another key issue discussed. Another Australian small-group trial of GP education did not show any effect on providing WAAPs.17 We believe the workshops in our study were effective because they included a combination of training in communication skills plus best-practice asthma management in a supportive learning environment.10
In comparison with the PACE USA trial, we found similar-sized increases in the proportion of doctors who provided written instructions about asthma therapy.13 Although we used the framework of the PACE USA intervention, the workshops in our study included more specific teaching about matching medication to pattern of asthma, the importance of providing WAAPs, and assessing device competency. GPs in the intervention group of our study improved their practice in each of these domains. Unlike the PACE USA trial, we did not find significant differences in GPs addressing fears about asthma medications. This may reflect differences in the participants (GPs rather than primary care paediatricians), the severity of asthma (“mild to moderate” in our study compared with “more severe” in the US study), or treatment concerns (possibly more anxiety about inhaled corticosteroid use in the US).
A limitation of our study was the use of data that were self-reported by participating GPs. However, the use of parent-reported outcomes (eg, receipt of a WAAP) provided strong validation of GP-reported outcomes. In addition, the length of the questionnaires, which were based on those used in the PACE USA trial,13 may have reduced the questionnaire response rate at 12 months. A potential limitation of our study was slow recruitment, which led to a need to recruit more than one GP per practice. However, this clustering had a negligible effect on the results.
Despite our success in recruiting GPs, we were only able to recruit 221 families with a child with asthma in the study period. While some GPs did not have paediatric asthma patients, a major barrier was that we were only able to contact parents who had replied to their GP’s invitation, a well recognised challenge.18
4 General practitioner-reported measures of asthma management
Asks parent or carer to demonstrate use of asthma device very often or always |
|||||||||||||||
Received 6 December 2010, accepted 14 July 2011
- Smita Shah1
- Susan M Sawyer2
- Brett G Toelle3
- Craig M Mellis4
- Jennifer K Peat5
- Marivic Lagleva1
- Timothy P Usherwood6
- Christine R Jenkins3
- 1 Primary Health Care Education and Research Unit, Westmead Hospital, University of Sydney, Sydney, NSW.
- 2 Department of Paediatrics, University of Melbourne, Melbourne, VIC.
- 3 Woolcock Institute of Medical Research, Sydney, NSW.
- 4 Central Clinical School, University of Sydney, Sydney, NSW.
- 5 School of Exercise Science, Australian Catholic University, Sydney, NSW.
- 6 Department of General Practice, Sydney Medical School — Westmead, University of Sydney, Sydney, NSW.
No relevant disclosures.
- 1. Australian Centre for Asthma Monitoring. Asthma in Australia 2008. Canberra: Australian Institute of Health and Welfare, 2008. (AIHW Asthma Series No. 3; AIHW Cat. No. ACM 14.)
- 2. Shah S, Roydhouse JK, Sawyer SM. Asthma education in primary healthcare settings. Curr Opin Pediatr 2008; 20: 705.
- 3. Dinakar C, Van Osdol TJ, Wible K. How frequent are asthma exacerbations in a pediatric primary care setting and do written asthma action plans help in their management? J Asthma 2004; 41: 807-812.
- 4. Gibson PG, Powell H. Written action plans for asthma: an evidence-based review of the key components. Thorax 2004; 59: 94.
- 5. Grol R, Wensing M. What drives change? Barriers to and incentives for achieving evidence-based practice. Med J Aust 2004; 180 (6 Suppl): S57-S60. <MJA full text>
- 6. Goeman DP, Hogan CD, Aroni RA, et al. Barriers to delivering asthma care: a qualitative study of general practitioners. Med J Aust 2005; 183: 457-460. <MJA full text>
- 7. Sawyer SM, Shah S. Improving asthma outcomes in harder-to-reach populations: challenges for clinical and community interventions. Paediatr Respir Rev 2004; 5: 207-213.
- 8. Barton C, Proudfoot J, Amoroso C, et al. Management of asthma in Australian general practice: care is still not in line with clinical practice guidelines. Prim Care Respir J 2009; 18: 100-105.
- 9. Roydhouse JK, Shah S, Toelle BG, et al. A snapshot of general practitioner attitudes, levels of confidence and self-reported paediatric asthma management practice. Aust J Primary Health 2011; 17: 288-293.
- 10. Shah S, Toelle BG, Sawyer SM, et al. Feasibility study of a communication and education asthma intervention for general practitioners in Australia. Aust J Prim Health 2010; 16: 75-80.
- 11. Glasgow N. Systems for the management of respiratory disease in primary care-an international series: Australia. Prim Care Respir J 2008; 17: 19-25.
- 12. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control of chronic disease. Health Educ Behav 2001; 28: 769-782.
- 13. Clark NM, Gong M, Schork MA, et al. Impact of education for physicians on patient outcomes. Pediatrics 1998; 101: 831-836.
- 14. Centre for Epidemiology and Research, New South Wales Department of Health. New South Wales child health survey 2001. N S W Public Health Bull 2002; 13 Suppl 3: 1-84.
- 15. Donner A, Klar N. Design and analysis of cluster randomization trials in health research. London: Arnold, 2000.
- 16. National Asthma Council Australia. Asthma management handbook 2006. Melbourne: National Asthma Council Australia, 2006.
- 17. Sulaiman ND, Barton CA, Liaw ST, et al. Do small group workshops and locally adapted guidelines improve asthma patients’ health outcomes? A cluster randomized controlled trial. Fam Pract 2010; 27: 246-254.
- 18. Hoddinott P, Britten J, Harrild K, Godden DJ. Recruitment issues when primary care population clusters are used in randomised controlled clinical trials: climbing mountains or pushing boulders uphill? Contemp Clin Trials 2007; 28: 232-241.





Abstract
Objective: To evaluate the effectiveness of the Practitioner Asthma Communication and Education (PACE) Australia program, an innovative communication and paediatric asthma management program for general practitioners.
Design: Randomised controlled trial.
Setting: General practices from two regions in metropolitan Sydney.
Participants: 150 GPs, who were recruited between 2006 and 2008, and 221 children with asthma in their care.
Intervention: GPs in the intervention group participated in two 3-hour workshops, focusing on communication and education strategies to facilitate quality asthma care.
Main outcome measures: Patient outcomes included receipt of a written asthma action plan (WAAP), appropriate medication use, parent days away from work, and child days away from school or child care. GP outcomes included frequency of providing a WAAP and patient education, communication and teaching behaviour, and adherence to national asthma guidelines regarding medication use.
Results: More patients of GPs in the intervention group reported receipt of a WAAP (difference, 15%; 95% CI, 2% to 28%; adjusted P = 0.046). In the intervention group, children with infrequent intermittent asthma symptoms had lower use of inhaled corticosteroids (difference, 24%; 95% CI, − 43% to − 5%; P = 0.03) and long-acting bronchodilators (difference, 19%; 95% CI, − 34% to − 5%; P = 0.02). GPs in the intervention group were more confident when communicating with patients (difference 22%; 95% CI, 3% to 40%; P = 0.03). A higher proportion of GPs in the intervention group reported providing a WAAP more than 70% of the time (difference, 23%; 95% CI, 11% to 36%; adjusted P = 0.002) and prescribing spacer devices more than 90% of the time (difference, 29%; 95% CI, 16% to 42%; adjusted P = 0.02).
Conclusions: The PACE Australia program improved GPs’ asthma management practices and led to improvements in some important patient outcomes.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12607000067471.