In 2009, acute coronary syndromes (ACS) accounted for about 80 000 hospital admissions and 10 000 deaths in Australia.1 Rising levels of obesity and diabetes in an ageing population suggest the number of events is likely to double by the year 2030.2 Randomised clinical trials have demonstrated the mortality benefit of pharmacological and procedural interventions for this condition, and cross-sectional studies have shown that better adherence to these therapies is effective in reducing mortality and morbidity.3,4 However, the application of therapeutic strategies across different regions varies.5
In Australia and New Zealand, gaps exist between guideline-recommended care and the inhospital application of recommended therapy.6,7 This information has been derived from registry studies of disparate populations of patients with ACS that used different recruitment strategies from different hospitals over varying periods of time and are not directly comparable.
The Global Registry of Acute Coronary Events (GRACE) recorded comprehensive information on ACS patients from a consistent cohort of Australian and New Zealand hospitals over an 8-year period (January 2000 to December 2007). A recent analysis of the global GRACE cohort, capturing data to 2005, reported a direct association between improvements in the management of patients with ACS and significant improvements in inhospital outcomes and outcomes at 6 months.8 We aimed to determine whether outcomes demonstrated for the global cohort as a whole are true for the patient cohort recruited from Australia and New Zealand and whether the trends towards improvement continued through the latter years of the registry.
Details of the GRACE methodology have been described previously.9
Participating Australian and New Zealand institutions were selected to encompass a range of facilities and geographical locations. Of the 11 centres contributing data, six were metropolitan centres, five had provision for 24-hour percutaneous coronary intervention (PCI) and four had onsite cardiac surgery. By the end of the recruitment phase in 2007, one regional centre had acquired a cardiac catheter laboratory with daytime PCI capabilities. One metropolitan centre had evolved from providing only diagnostic angiography to a 24-hour, 7-day-per-week interventional service. Six of the 11 institutions provided data to the registry throughout the entire enrolment period.
Patients enrolled during 2000–2007 who had a discharge diagnosis of either ST-segment-elevation myocardial infarction (STEMI), including new left-bundle branch block, or non-ST-segment-elevation ACS (NSTEACS), including non-STEMI and unstable angina, were included in this analysis. Outcomes reported include inhospital death, recurrent myocardial infarction, stroke, heart failure, cardiogenic shock and fatal or life-threatening bleeding events. Surviving patients were contacted 6 months after discharge to establish the incidence of death, stroke, myocardial infarction and readmission for ischaemic heart disease.
In Australia and New Zealand, participants’ informed consent was required before the extraction of their medical information and to facilitate follow-up. In January 2002, the ethics committees of participating institutions agreed to waive consent in one circumstance — so that eligible patients who died before they could be approached for participation in the registry could be enrolled.
Categorical data are presented as frequencies and percentages, and means and SDs are provided for continuous variables. The double-sided Cochran-Armitage test was used to evaluate time trends at a significance level of α = 0.05. Analyses were carried out using SAS (SAS Institute Inc, Cary, NC, USA), and SPSS software, version 16.0 (SPSS Inc, Chicago, Ill, USA). GRACE risk scores were calculated as previously reported.10
Data were greater than 97% complete for all variables, with the exception of Killip Class, which was complete for 93.7% of patients. Six-month follow-up data and outcomes are reported for all patients, including those who were enrolled before the consent waiver was applied in 2002.
From 2002 to 2007, a total of 5615 patients with a diagnosis of STEMI (1723) or NSTEACS (3892) were recruited from nine Australian centres and two New Zealand centres. The age and sex distribution of the patients did not change over time (Box 1). Although there were some variations in baseline characteristics, the GRACE risk score predicting in-hospital mortality did not change in either cohort over time.
From 2000–2007 among patients with STEMI, our data showed a 15.6% decline in the use of unfractionated heparin (P < 0.0001) and a non-significant 5.3% increase in the use of low-molecular-weight heparin (Box 2). Use of glycoprotein (GP) IIb/IIIa receptor antagonists increased by 6.3% during 2000–2005, after which the rate of use declined by the end of 2007, returning to a similar rate of use as in 2000 (Box 2). Treatment with aspirin remained high throughout 2000–2007. Prescription of a thienopyridine derivative (clopidogrel or ticlopidine) increased significantly from 27.6% in 2000 to 80.4% in 2007 (P < 0.0001). This rise was influenced by an increase in the use of thienopyridines for medically managed patients (P < 0.0001). Prescription of statin therapy increased by 24.0% overall (P < 0.0001), and prescription of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) increased by 13.3% (P < 0.0001). There was no significant change in the prescription of β-blockers during this period (Box 2).
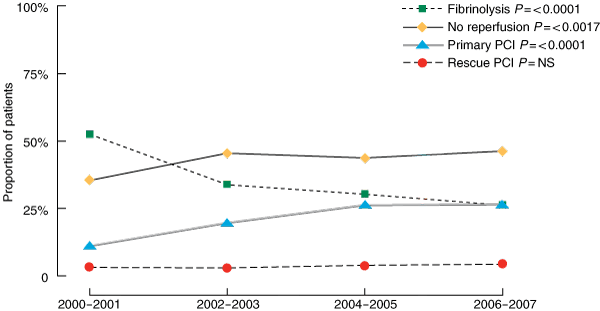
Reperfusion with primary PCI increased by 15% (P < 0.0001), accompanied by an overall decrease in fibrinolytic therapy of 26% (Box 3). The rates of rescue PCI (for failed thrombolysis) remained constant (Box 3). Overall coronary angiography rates during admission increased by 15.0% (P < 0.0001) and overall PCI rates increased by 17.0% (P < 0.0001) while coronary artery bypass graft (CABG) surgery rates remained unchanged (Box 2).
Overall, data from 2000–2007 that did not include patients who died soon after admission during the early years of the registry showed no change in inhospital mortality (Box 4). After the introduction of the consent waiver in 2002, collection of more complete mortality data was possible. Between 2002 and 2007, there was a 3.9% reduction in in-hospital deaths (P = 0.04), and a significant decline in stroke (P = 0.004), although the numbers reported for stroke were small (Box 4). There was an 8.2% reduction in congestive heart failure events in hospital (P = 0.003), and there was no change in fatal or life-threatening bleeding event rates or episodes of acute renal failure (Box 4). Among the cohort of patients surviving to hospital discharge, the incidences of death and stroke at 6-month follow-up did not change between 2000 and 2007; there was, however, a 4.2% reduction in rates of reported MI (P = 0.01) and a 13.1% reduction in hospital readmissions for ischaemic heart disease (P < 0.0001).
From 2000 to 2007 among people with NSTEACS, the inhospital use of unfractionated heparin declined by 20.2% (P < 0.0001) but use of low-molecular-weight heparin did not change (P = 0.33) (Box 2). Use of GP IIb/IIIa receptor antagonists fluctuated between 2000 and 2007, with a peak use in 10.1% of participants during 2004–2005, and then a fall to 7.0% during 2006–2007. The overall trend was significant (P = 0.01) (Box 2). Thienopyridine use increased by 43.6% (P < 0.0001) overall, and by 37.4% in patients who did not undergo PCI (P < 0.0001). Prescription of aspirin remained consistently high, β-blocker therapy increased by about 10% (P = 0.01) and the prescription of both statin and ACE inhibitors or ARBs increased by about 20% (P < 0.0001 for both) (Box 2).
Throughout 2000–2007, inhospital mortality rates for patients with NSTEACS were low (Box 4). When the analysis was limited to patients who were recruited after the consent waiver in 2002, there was a 1.8% reduction in inhospital mortality (P = 0.04). There was also a significant reduction in episodes of heart failure for this period (P = 0.0001). Inhospital stroke and MI rates remained unchanged. Outcomes at 6 months following hospital discharge revealed an overall fall in the rates of death (P = 0.02), stroke (P = 0.001) and an 8.7% reduction in hospital readmission rates for ischaemic heart disease (P < 0.0001) (Box 4).
Our analysis of data from this large cohort of patients provides evidence of substantial improvement in outcomes in Australian and New Zealand patients with ACS over an 8-year period from 2000 to 2007. This was accompanied by an increase in the uptake of evidence-based medical therapies.
In Australia and New Zealand there has been limited opportunity to describe and follow management and outcomes in well defined ACS populations. This reflects a lack of organised state and national registries coupled with limited funding for clinical data collection at an institutional level. Our analysis represents the first opportunity to evaluate changes in practice patterns and outcomes in a well defined population of ACS patients in Australia and New Zealand over time, and is the largest cohort to date.
The local cohort was limited to 11 participating institutions, restricting the extrapolation of results across either country as a whole. However, our data are very similar to those from other national ACS registries that recruited patients during this period. Additionally, it is likely that unmeasured pre- and posthospital factors, such as improved primary and secondary risk-factor management in the Australian and New Zealand populations as a whole, contributed to improvement in outcomes, and this information was not collected in the hospital-based GRACE registry.
During the 8 years of data collection for this study there were significant changes in patient management, particularly in the uptake of specific evidence-based medical therapies. The use of thienopyridines increased incrementally over time, predominantly in medically managed patients. Our data showed a significant increase in the use of statin therapy among patients with STEMI and patients with NSTEACS following outcomes of secondary prevention trials coupled with more recent studies advocating the benefits of commencing lipid-lowering therapy early during hospitalisation for an ACS.11-14
The implementation of all evidence-based guideline-recommended therapies among patients in our study was non-uniform, in particular, the use of intravenous heparin and GP IIb/IIIa receptor-antagonist therapy, which fell among patients with NSTEACS after 2005. The low use of GP IIb/IIIa therapy has been a consistent feature of Australian and New Zealand practice, despite local guidelines supporting its use.7,15,16 The lack of enthusiasm for GP IIb/IIIa therapy may be driven by cost constraints, together with concerns that the reduction in cardiovascular events is offset by an increased bleeding risk in the presence of combination oral antiplatelet therapy.7,16 This observation serves to highlight the reality of physicians applying the guidelines selectively as they use their clinical judgement when choosing appropriate therapies for their patients.17,18 The factors that influence this decision-making process warrant further study.
One of the main practice changes in the management of STEMI in urban centres has been the evolution from fibrinolysis as the predominant treatment to primary PCI.19,20 Among the Australian and New Zealand cohort we found a 26% reduction in fibrinolysis, accompanied by only a 15% increase in primary PCI. This apparent fall in total reperfusion may in fact be artefactual. A significant proportion of STEMI patients (10%–25%), who according to earlier accepted practice would have received fibrinolysis, undergo angiography and turn out to have minor coronary disease or disease that is not amenable to percutaneous revascularisation, and therefore do not receive PCI.21,22 As recruitment ended in 2007, there may have been insufficient time to determine the impact of additional access to PCI on the absolute increase in PCI rates and the corresponding decrease in fibrinolysis.
A second notable observation from our analysis was the modest increment in coronary angiography and PCI offered to patients with NSTEACS, with no increase in CABG surgery during the study period. This failure to offer coronary angiography and subsequent revascularisation may be due in part to clinicians’ uncertainty of the risk versus the benefit of undergoing an interventional procedure, particularly for higher-risk patients who are not well represented in the randomised trials.18 It would seem that this concern is unfounded, as multiple lines of evidence suggest the higher the stratified risk for an ACS, the greater the benefit from an invasive strategy.23,24
Despite limited application of evidence-guided therapies during the 8 years of data collection for this study, we observed significantly reduced inhospital heart failure episodes among patients with STEMI and patients with NSTEACS. These data are not implausible and reflect findings in the overall GRACE cohort.8 A recent Australian Institute of Health and Welfare (AIHW) analysis reported a 20% decline in hospital admission among patients with heart failure between the financial years 1996–07 and 2003–04, thought to be due, among other things, to improvements in heart failure treatment.25 It is plausible that improvements in revascularisation, together with increased use of ACE inhibitors, are contributing to reduce acute heart failure among patients with ACS.
Additionally, with the collection of more complete mortality data from 2002 onward, a fall in hospital mortality was evident in both groups of patients. These mortality data are consistent with a recent Access Economics report derived from AIHW data suggesting a fall in 28-day mortality for ACS patients in Australia of about 0.5% per year from 2004 to 2009.1 Among patients with STEMI or NSTEACS who survived to hospital discharge and were contacted after 6 months, there was a reduction in hospital readmissions for ischaemic heart disease, and a fall in stroke among those with NSTEACS. These data point to an overall salutary effect associated with the improvement in the quality of care offered to contemporary ACS patients in Australia and New Zealand.
Further study is required to evaluate whether the improvements in therapies are appropriately directed towards the highest-risk patients. Important practice gaps remain, particularly in the provision of emergency reperfusion for STEMI and revascularisation for NSTEACS. Future efforts should be directed toward identification of the patient-, clinician- and system-related factors that impede practice improvement in these areas.
1 Characteristics for patients treated for STEMI and NSTEACS between 2000 and 2007, at baseline and at the end of the study period
|
STEMI* |
NSTEACS* |
|||||||||||||
|
2000–2001 (n = 533) |
2006–2007 (n = 306) |
P† |
2000–2001 (n = 1069) |
2006–2007 (n = 800) |
P† |
|||||||||
Demographics |
|
|
|
|
|
|
|||||||||
Men |
375 (70.4%) |
233 (76.1%) |
0.07 |
718 (67.2%) |
534 (66.7%) |
0.78 |
|||||||||
Age, mean |
64.1 |
63.2 |
0.30 |
64.1 |
63.2 |
0.33 |
|||||||||
Medical history |
|
|
|
|
|
|
|||||||||
Prior MI |
93 (17.5%) |
59 (19.3%) |
0.51 |
449 (42.0%) |
318 (39.8%) |
0.37 |
|||||||||
Prior CHF |
34 (6.4%) |
20 (6.5%) |
0.93 |
143 (13.4%) |
101 (12.6%) |
0.61 |
|||||||||
Angina |
208 (39.0%) |
64 (20.9%) |
< 0.0001 |
746 (70.0%) |
419 (52.4%) |
< 0.0001 |
|||||||||
Prior CABG surgery |
25 (4.7%) |
19 (6.2%) |
0.33 |
233 (21.8%) |
144 (18.0%) |
0.04 |
|||||||||
Prior PCI |
37 (7%) |
21 (6.9%) |
0.96 |
186 (17.4%) |
175 (22.0%) |
0.01 |
|||||||||
Hypertension |
246 (46.2%) |
156 (50.9%) |
0.17 |
616 (57.6%) |
509 (63.6%) |
0.005 |
|||||||||
Diabetes (type 1 or 2) |
107 (20.1%) |
68 (22.2%) |
0.47 |
280 (26.2%) |
235 (29.4%) |
0.12 |
|||||||||
Hyperlipidaemia |
219 (41.1%) |
140 (46.0%) |
0.16 |
627 (58.7%) |
519 (65.0%) |
0.006 |
|||||||||
Renal impairment |
18 (3.4%) |
13 (4.2%) |
0.51 |
77 (7.2%) |
64 (8.0%) |
0.50 |
|||||||||
Smoker (current) |
169 (31.7%) |
97 (31.7%) |
0.64 |
211 (19.7%) |
153 (19.1%) |
0.01 |
|||||||||
Smoker (ex) |
345 (64.7%) |
188 (61.4%) |
0.28 |
664 (62.2%) |
488 (61.0%) |
0.45 |
|||||||||
Prior TIA/stroke |
51 (9.6%) |
19 (6.2%) |
0.08 |
127 (12.0%) |
106 (13.3%) |
0.39 |
|||||||||
PVD |
40 (7.5%) |
16 (5.2%) |
0.19 |
118 (11%) |
51 (6.4%) |
0.0005 |
|||||||||
Index ECG result |
|
|
|
|
|
|
|||||||||
ST elevation |
462 (86.7%) |
249 (81.4%) |
0.03 |
54 (5.1%) |
32 (4.0%) |
0.28 |
|||||||||
LBBB |
31 (5.8%) |
30 (9.8%) |
0.03 |
86 (8.0%) |
43 (5.4%) |
0.02 |
|||||||||
Abnormal for ischaemia |
511 (95.9%) |
293 (95.8%) |
0.93 |
696 (65.1%) |
439 (54.9%) |
< 0.0001 |
|||||||||
ST deviation (non-specific) |
478 (89.7%) |
265 (86.6%) |
0.17 |
291 (27.2%) |
204 (25.5%) |
0.40 |
|||||||||
ST depression |
265 (49.7%) |
125 (41.0%) |
0.01 |
245 (22.9%) |
181 (22.6%) |
0.88 |
|||||||||
T wave inversion |
158 (29.6%) |
51 (16.7%) |
< 0.0001 |
338 (31.6%) |
202 (25.3%) |
0.002 |
|||||||||
Physical characteristic |
|
|
|
|
|
|
|||||||||
Systolic BP, mean (SD) |
139.5 (28.6) |
140.5 (30.8) |
0.64 |
139 (28.6) |
140 (30.8) |
0.64 |
|||||||||
Diastolic BP, mean (SD) |
81.4 (18.1) |
81.9 (19.1) |
0.73 |
81.4 (18.1) |
81.9 (19.0) |
0.70 |
|||||||||
Heart rate, mean (SD) |
78.5 (21.0) |
79.9 (30.8) |
0.38 |
78.5 (21.0) |
79.9 (23.3) |
0.38 |
|||||||||
Killip Class I |
409 (77.8%) |
224 (81.8%) |
0.13 |
823 (77.6%) |
611 (83.7%) |
0.0014 |
|||||||||
Killip Class II–IV |
117 (22.2%) |
50 (18.2%) |
0.18 |
238 (22.4%) |
119 (16.3%) |
0.0014 |
|||||||||
Cardiac arrest |
22 (4.2%) |
12 (4.0%) |
0.88 |
7 (0.7%) |
13 (1.6%) |
0.04 |
|||||||||
Laboratory test |
|
|
|
|
|
|
|||||||||
Positive initial cardiac enzymes |
251 (47.1%) |
199 (65.9%) |
< 0.0001 |
319 (30.0%) |
361 (45.4%) |
< 0.0001 |
|||||||||
Serum creatinine (μmol/L), mean (SD) |
81 (53.0) |
97 (44.0) |
< 0.0001 |
81 (53.0) |
103 (44.0) |
< 0.0001 |
|||||||||
Serum cholesterol (mmol/L), mean (SD) |
5.3 (1.2) |
4.9 (1.2) |
0.0002 |
5.3 (1.2) |
4.9 (1.2) |
< 0.0001 |
|||||||||
GRACE risk score‡ (SD) |
139.6 (35.7) |
139.1 (36.9) |
0.18 |
121.7 (35.6) |
122 (35.7) |
0.15 |
|||||||||
Data in italics were calculated using a different denominator owing to missing data. BP = blood pressure. CABG = coronary artery bypass graft. CHF = congestive heart failure. ECG = electrocardiogram. GRACE = Global Registry of Acute Coronary Events. LBBB = left-bundle branch block. MI = myocardial infarction. NSTEACS = non-ST-segment-elevation myocardial infarction. PCI = percutaneous coronary intervention. PVD = peripheral vascular disease. STEMI = ST-segment-elevation myocardial infarction. TIA = transient ischaemic attack. * Figures are no. (%) unless otherwise stated. † P < 0.05 significant. ‡ For inhospital morbidity. |
|||||||||||||||
2 Changes in medical therapy for patients treated for STEMI and NSTEACS, 2000–2007
|
Total no. of patients (%) |
||||||||||||||
|
2000–2001 |
2002–2003 |
2004–2005 |
2006–2007 |
P for trend* |
||||||||||
STEMI |
n = 533 |
n = 369 |
n = 354 |
n = 306 |
|
||||||||||
Aspirin (excl CI) |
501 (94.0%) |
333 (90.2%) |
337 (96.3%) |
291 (95.0%) |
0.43 |
||||||||||
β-blocker (excl CI) |
415 (78.0%) |
290 (78.5%) |
259 (73.0%) |
259 (85.0%) |
0.60 |
||||||||||
Statin |
344 (64.5%) |
295 (80%) |
314 (89.0%) |
269 (88.5%) |
< 0.0001 |
||||||||||
ACE inhibitor/ARB |
355 (67.0%) |
275 (74.5%) |
274 (77.8%) |
237 (80.3%) |
< 0.0001 |
||||||||||
Total LMWH |
241 (45.5%) |
193 (52.3%) |
180 (51.5%) |
155 (50.8%) |
0.13 |
||||||||||
UFH |
377 (71.5%) |
235 (63.7%) |
213 (60.3%) |
170 (55.9%) |
< 0.0001 |
||||||||||
Thienopyridine (total) |
146 (27.6%) |
207 (56.1%) |
248 (70.3%) |
246 (80.4%) |
< 0.0001 |
||||||||||
Thienopyridine (without PCI) |
14 (2.6%) |
65 (17.6%) |
86 (24.3%) |
106 (34.6%) |
< 0.0001 |
||||||||||
GP IIb/IIIa receptor antagonists |
111 (20.8%) |
97 (26.3%) |
96 (27.1%) |
68 (22.0%) |
0.06 |
||||||||||
Calcium channel blockers |
59 (11.1%) |
32 (8.7%) |
20 (5.6%) |
32 (10.5%) |
0.10 |
||||||||||
Coronary angiography |
324 (60.8%) |
255 (69.1%) |
288 (81.4%) |
232 (75.8%) |
< 0.0001 |
||||||||||
PCI (all) |
192 (36.0%) |
169 (45.8%) |
204 (57.6%) |
162 (53.0%) |
< 0.0001 |
||||||||||
CABG surgery |
48 (9.0%) |
46 (12.5%) |
28 (7.9%) |
19 (6.2%) |
0.09 |
||||||||||
NSTEACS |
n = 1069 |
n = 849 |
n = 811 |
n = 800 |
|
||||||||||
Aspirin (excl CI) |
954 (89.2%) |
712 (83.8%) |
731 (90.1%) |
732 (91.5%) |
0.11 |
||||||||||
β-blocker (excl CI) |
772 (72.2%) |
652 (76.8%) |
653 (80.6%) |
654 (81.8%) |
0.01 |
||||||||||
Statin |
699 (65.4%) |
684 (80.6%) |
690 (85.1%) |
683 (84.4%) |
< 0.0001 |
||||||||||
ACE/ARB antagonist |
557 (52.1%) |
512 (60.3%) |
523 (64.5%) |
574 (71.8%) |
< 0.0001 |
||||||||||
Total LMWH |
768 (71.8%) |
635 (74.8%) |
581 (71.6%) |
595 (74.4%) |
0.33 |
||||||||||
UFH |
449 (42.0%) |
309 (36.4%) |
239 (29.5%) |
174 (21.8%) |
< 0.0001 |
||||||||||
Thienopyridine (total) |
209 (19.5%) |
378 (44.5%) |
432 (53.3%) |
505 (63.1%) |
< 0.0001 |
||||||||||
Thienopyridine (without PCI) |
72 (6.7%) |
225 (26.5%) |
258 (31.8%) |
353 (44.1%) |
< 0.0001 |
||||||||||
GP IIb/IIIa receptor antagonists |
69 (6.5%) |
69 (8.1%) |
82 (10.1%) |
56 (7.0%) |
0.01 |
||||||||||
Calcium channel blockers |
318 (29.8%) |
185 (21.8%) |
203 (25.0%) |
209 (26.1%) |
0.01 |
||||||||||
Coronary angiography |
574 (53.7%) |
500 (58.9%) |
491 (60.5%) |
461 (57.6%) |
0.03 |
||||||||||
PCI |
212 (19.8%) |
234 (27.6%) |
222 (27.4%) |
202 (25.3%) |
0.004 |
||||||||||
CABG surgery |
120 (11.2%) |
114 (13.4%) |
101 (12.5%) |
79 (9.9%) |
0.39 |
||||||||||
Total revascularisation |
337 (31.5%) |
345 (40.6%) |
321 (39.6%) |
287 (35.9%) |
0.04 |
||||||||||
Data in italics were calculated using a different denominator owing to missing data. ACE = angiotensin-converting enzyme. ARB = angiotensin-II receptor blocker. CABG = coronary artery bypass graft. Excl CI = excluding contraindications. GP = glycoprotein. LMWH = low-molecular-weight heparin. PCI = percutaneous coronary intervention. NSTEACS = non-ST-segment-elevation acute coronary syndrome. STEMI = ST-segment-elevation myocardial infarction. UFH = unfractionated heparin. * P < 0.05 significant. |
|||||||||||||||
3 Temporal trends in reperfusion therapy for patients with ST-segment-elevation myocardial infarction from Jan 2000 to Dec 2007
 | |||||||||||||||
|
PCI = percutaneous coronary intervention. | |||||||||||||||
4 Changes in clinical outcomes for patients with STEMI and NSTEACS, 2000–2007
|
Total no. of patients (%) |
||||||||||||||
|
2000–2001 |
2002–2003 |
2004–2005 |
2006–2007 |
P for trend* |
||||||||||
STEMI |
n = 529 |
n = 369 |
n = 354 |
n = 306 |
|
||||||||||
Inhospital death |
20 (3.8%) |
33 (8.9%) |
26 (7.4%) |
15 (5.0%) |
0.32 |
||||||||||
Stroke |
4 (0.8%) |
9 (2.4%) |
4 (1.1%) |
0 |
0.32 |
||||||||||
Cardiogenic shock |
18 (3.4%) |
25 (7.0%) |
25 (7.1%) |
12 (4.0%) |
0.36 |
||||||||||
MI > 24hrs after presentation |
0 |
14 (3.8%) |
8 (2.3%) |
13 (4.2%) |
0.20 |
||||||||||
CHF/pulmonary oedema |
111 (21.0%) |
76 (20.6%) |
47 (13.3%) |
38 (12.4%) |
0.0002 |
||||||||||
Renal failure |
23 (4.3%) |
21 (5.7%) |
19 (5.4%) |
16 (5.2%) |
0.52 |
||||||||||
Major bleeding event† |
7 (1.3%) |
8 (2.2%) |
7 (2.0%) |
3 (1.0%) |
0.88 |
||||||||||
Outcomes from discharge to 6 months |
|||||||||||||||
Death |
23 (4.3%) |
12 (3.3%) |
5 (1.4%) |
9 (2.9%) |
0.13 |
||||||||||
Stroke |
11 (2.1%) |
4 (1.1%) |
1 (0.3%) |
3 (1.1%) |
0.08 |
||||||||||
MI |
22 (5.2%) |
10 (3.3%) |
7 (2.3%) |
5 (1.0%) |
0.01 |
||||||||||
Readmission for IHD |
119 (22.5%) |
73 (19.8%) |
48 (13.5%) |
29 (9.4%) |
< 0.0001 |
||||||||||
NSTEACS |
n = 1045 |
n = 849 |
n = 811 |
n = 800 |
|
||||||||||
Inhospital death |
12 (1.1%) |
35 (4.1%) |
34 (4.2%) |
18 (2.3%) |
0.06 |
||||||||||
Stroke |
5 (0.5%) |
1 (0.1%) |
5 (0.6%) |
1 (0.1%) |
0.52 |
||||||||||
Cardiogenic shock |
5 (0.5%) |
11 (1.3%) |
22 (2.7%) |
10 (1.3%) |
0.01 |
||||||||||
MI > 24 hrs after presentation |
0 |
6 (0.7%) |
7 (0.9%) |
9 (1.1%) |
0.92 |
||||||||||
CHF/pulmonary oedema |
138 (13.2%) |
78 (9.2%) |
62 (7.6%) |
33 (4.1%) |
0.0001 |
||||||||||
Renal failure |
20 (1.9%) |
30 (3.5%) |
31 (3.8%) |
20 (2.5%) |
0.25 |
||||||||||
Major bleeding event |
13 (1.2%) |
6 (0.7%) |
9 (1.1%) |
7 (0.9%) |
0.62 |
||||||||||
Outcomes from discharge to 6 months |
|||||||||||||||
Death |
57 (5.5%) |
30 (3.5%) |
18 (2.2%) |
22 (2.8%) |
0.02 |
||||||||||
Stroke |
21 (2.0%) |
9 (1.1%) |
6 (0.7%) |
3 (0.4%) |
0.001 |
||||||||||
MI |
37 (3.5%) |
33 (3.9%) |
24 (3.0%) |
30 (3.8%) |
0.83 |
||||||||||
Readmission for IHD |
249 (23.8%) |
163 (19.2%) |
132 (16.3%) |
121 (15.1%) |
< 0.0001 |
||||||||||
Data in italics were calculated using a different denominator owing to missing data. CHF = congestive heart failure. CV = cardiovascular. IHD = ischaemic heart disease. MI = myocardial infarction. NSTEACS = non-ST-segment-elevation acute coronary syndrome. STEMI = ST-segment-elevation myocardial infarction. * P < 0.05 significant. † Fatal or life-threatening. |
|||||||||||||||
Received 21 November 2010, accepted 20 April 2011
- Bernadette Aliprandi-Costa1
- Isuru Ranasinghe1,2
- Vincent Chow1
- Shruti Kapila3
- Craig Juergens3
- Gerard Devlin4
- John Elliott5
- Jeff Lefkowitz6
- David B Brieger1
- 1 Department of Cardiology, Concord Repatriation General Hospital, Sydney, NSW.
- 2 Concord Clinical School, University of Sydney, Sydney, NSW.
- 3 Department of Cardiology, Liverpool Hospital, Sydney, NSW.
- 4 Waikato Hospital, Hamilton, New Zealand.
- 5 Christchurch Hospital, Christchurch, New Zealand.
- 6 Royal Melbourne Hospital, Melbourne, VIC.
We thank all participating GRACE ANZ Investigators and coordinators. GRACE is funded by an unrestricted educational grant from Sanofi-Aventis (Paris, France) to the Center for Outcomes Research, University of Massachusetts Medical School. Sanofi-Aventis had no involvement in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the paper for publication.
Craig Juergens has received payment from Eli Lilly for sitting on their advisory board and for travel expenses to international meetings. David Brieger has received unrestricted educational grants as well as payments from Eli Lilly, AstraZeneca, Sanofi-Aventis and Boehringer Ingelheim for sitting on their advisory boards and for lectures. He has also received an unrestricted educational grant from Merck/Schering-Plough.
- 1. Access Economics. The economic costs of heart attack and chest pain (acute coronary syndrome). Jun 2009. http://www.accesseconomics.com.au/publicationsreports/search. php?searchby=year&searchfor=2009 (accessed May 2011).
- 2. Senes S, Penm E. Medicines for cardiovascular health: are they used appropriately. Canberra: Australian Institute of Health and Welfare, 2007. (AIHW Cat. No. CVD 36; Cardiovascular Disease Series No. 27.)
- 3. Mukherjee D, Fang J, Chetcuti S, et al. Impact of combination evidence-based medical therapy on mortality in patients with acute coronary syndromes. Circulation 2004; 109: 745-749.
- 4. Peterson ED, Bynum DZ, Roe MT. Lessons learned from the CRUSADE National Quality Improvement Initiative. Curr Cardiol Rep 2008; 10: 285-290.
- 5. Chew DP, Amerena J, Coverdale S, et al. Current management of acute coronary syndromes in Australia: observations from the acute coronary syndromes prospective audit. Intern Med J 2007; 37: 741-748.
- 6. Walters DL, Aroney CN, Chew DP, et al. Variations in the application of cardiac care in Australia. Med J Aust 2008; 188: 218-223. <MJA full text>
- 7. Fox KA, Steg PG, Eagle KA, et al. Decline in rates of death and heart failure in acute coronary syndromes, 1999-2006. JAMA 2007; 297: 1892-1900.
- 8. Fox KA, Goodman SG, Anderson FA Jr, et al. From guidelines to clinical practice: the impact of hospital and geographical characteristics on temporal trends in the management of acute coronary syndromes. The Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2003; 24: 1414-1424.
- 9. Granger CB. Strategies of patient care in acute coronary syndromes: rationale for the Global Registry of Acute Coronary Events (GRACE) registry. Am J Cardiol 2000; 86: 4M-9M.
- 10. Granger CB, Goldberg RJ, Dabbous O, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003; 163: 2345-2353.
- 11. Manzato E. Scandinavian simvastatin study (4S). Lancet 1994; 344: 1767; author reply 1767-1768.
- 12. Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285: 1711-1718.
- 13. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344: 1383-1389.
- 14. Newby LK, Kristinsson A, Bhapkar MV, et al. Early statin initiation and outcomes in patients with acute coronary syndromes. JAMA 2002; 287: 3087-3095.
- 15. Aroney CN, Aylward P, Chew DP, et al. 2007 addendum to the National Heart Foundation of Australia/Cardiac Society of Australia and New Zealand Guidelines for the management of acute coronary syndromes 2006. Med J Aust 2008; 188: 302-303. <MJA full text>
- 16. Huynh LT, Chew DP, Sladek RM, et al. Unperceived treatment gaps in acute coronary syndromes. Int J Clin Pract 2009; 63: 1456-1464.
- 17. ST-elevation myocardial infarction: New Zealand management guidelines. N Z Med J 2005; 118: U1679.
- 18. Fox KA, Goodman SG, Klein W, et al. Management of acute coronary syndromes. Variations in practice and outcome; findings from the Global Registry of Acute Coronary Events (GRACE). Eur Heart J 2002; 23: 1177-1189.
- 19. Magid DJ, Calonge BN, Rumsfeld JS, et al. Relation between hospital primary angioplasty volume and mortality for patients with acute MI treated with primary angioplasty vs thrombolytic therapy. JAMA 2000; 284: 3131-3138.
- 20. Cannon CP, Gibson CM, Lambrew CT, et al. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA 2000; 283: 2941-2947.
- 21. Carstensen S, Nelson GC, Hansen PS, et al. Field triage to primary angioplasty combined with emergency department bypass reduces treatment delays and is associated with improved outcome. Eur Heart J 2007; 28: 2313-2319.
- 22. Hutchison AW, Malaiapan Y, Jarvie I, et al. Prehospital 12-lead ECG to triage ST-elevation myocardial infarction and emergency department activation of the infarct team significantly improves door-to-balloon times: ambulance Victoria and MonashHEART Acute Myocardial Infarction (MonAMI) 12-lead ECG project. Circ Cardiovasc Interv 2009; 2: 528-534.
- 23. TACTICS-TIMI 18 shows positive results of invasive approach. Cardiovasc J S Afr 2001; 12: 58-59.
- 24. Fox KA, Poole-Wilson PA, Henderson RA, et al; Randomized Intervention Trial of unstable Angina investigators. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Lancet 2002; 360: 743-751.
- 25. Najafi F, Dobson AJ, Jamrozik K. Recent changes in heart failure hospitalisations in Australia. Eur J Heart Fail 2007; 9: 228-233.





Abstract
Objectives: To describe temporal trends in the use of evidence-based medical therapies and management of patients with acute coronary syndromes (ACS) in Australia and New Zealand.
Design, setting and participants: Our analysis of the Australian and New Zealand cohort of the Global Registry of Acute Coronary Events (GRACE) included patients with ST-segment-elevation myocardial infarction (STEMI) and non-ST-segment-elevation ACS (NSTEACS) enrolled continuously between January 2000 and December 2007 from 11 metropolitan and rural centres in Australia and New Zealand.
Results: 5615 patients were included in this analysis (1723 with STEMI; 3892 with NSTEACS). During 2000–2007 there was an increase in the use of statin therapy, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and thienopyridines (P < 0.0001 for each). Among patients with STEMI, there was an increase in emergency revascularisation with PCI (from 11% to 27% [P < 0.0001]), and inhospital coronary angiography (from 61% to 76% [P < 0.0001]). Among patients with NSTEACS, there was an increase in revascularisation with PCI (from 20% to 25% [P = 0.004]). Heart failure rates declined substantially among STEMI and NSTEACS patients (from 21% to 12% [P = 0.0002], and from 13% to 4% [P < 0.0001], respectively) as did rates of hospital readmission for ischaemic heart disease at 6 months (from 23% to 9% [P = 0.0001], and from 24% to 15% [P = 0.0001], respectively).
Conclusions: From 2000 to 2007 in Australia and New Zealand, there was a fall in inhospital events and 6-month readmissions among patients admitted with ACS. This showed an association with improved uptake of guideline-recommended medical and interventional therapies. These data suggest an overall improvement in the quality of care offered to contemporary ACS patients in Australia and New Zealand.