Since around 1990, laparoscopic surgery has revolutionised abdominal surgery. Procedures such as laparoscopic cholecystectomy and Nissen fundoplication were quickly adopted and their use diffused rapidly through health systems around the world.1 However, the widespread adoption of laparoscopic surgery for colorectal cancer (CRC) has been much slower despite proven short-term benefits over open resection. These benefits included less blood loss, reduced pain, shorter postoperative ileus, reduced hospital stay and better postoperative pulmonary function.2 Specifically, time to first bowel movement is 1 day shorter with laparoscopic resection compared with open resection (3.5 v 4.5 days; P = 0.01), and length of hospital stay is reduced by about 3 days (8.1 v 11.8 days; P = 0.01).3
Reasons for the delay in uptake of laparoscopic surgery for CRC included less than optimal equipment and the long learning curve associated with the procedure; but perhaps most importantly, there were concerns about whether long-term oncological outcomes (eg, recurrence, overall survival) were as good as for open surgery.4
These concerns have subsequently been allayed. For example, a recent meta-analysis reported that across seven studies, only three of 826 patients with colon cancer (0.4%) who were randomly allocated to laparoscopic surgery had port-site metastases,5 and a Cochrane review concluded that resection with laparoscopic access resulted in cancer-related mortality equivalent to that for open surgery.5 Data from randomised trials in rectal cancer are not as mature,5 but the evidence that is available suggests that the oncological outcomes are equivalent for laparoscopic and open access surgery.6
We obtained data for eight financial years, 2000–01 to 2007–08, from the National Hospital Morbidity Database (NHMD), in which administrative inpatient data from all of the Australian states and territories is collated.7 The data extraction was restricted to patients with CRC who had had an elective surgical resection. Patients who had undergone emergency resections (eg, for bowel obstruction, bleeding or perforation secondary to CRC) were excluded because emergency surgery for complications of CRC and elective surgery for CRC are two distinct and different groups of surgical procures. Currently, laparoscopic surgery is only routinely considered for elective surgery — randomised controlled trials of laparoscopic surgery include only elective cases.
The NHMD uses Australian Classification of Health Interventions (ACHI) codes (International statistical classification of diseases and related health problems. 10th revision, Australian modification; ICD-10-AM),8 which are derived from the Medicare Benefits Schedule to indicate the procedure performed. There are currently no specific item numbers for laparoscopic resection of the colon or rectum. Instead, item numbers for laparoscopy (3039000 and 3039300) are used in combination with those for open resection of the colon or rectum to indicate a laparoscopic resection (Box 1).8
To simplify and clarify the presentation of results, we grouped procedures into two categories: those for elective segmental resection of the colon and those for elective resection of the rectum (Box 1). Total colectomy and proctocolectomy were not included in the data extracted because they are uncommon. Cases of Hartmann’s procedure were also excluded from the data extracted because, in Australia, this is typically an emergency procedure.
There were 5424 elective segmental resections for colon cancer in Australian hospitals in 2000–01 and 6523 in 2007–08; elective resections for rectal cancer increased from 2530 to 3072 in the same period. There was no change in the percentage of elective resections for CRC done in public versus private hospitals between 2000–01 and 2007–08. However, within the private sector, an increasing percentage of resections were performed in low-volume compared with high-volume hospitals. For public hospitals, the percentage of resections performed in low-volume compared with high-volume hospitals remained about the same (Box 2).
Over the 8 years of the study, the percentage of elective resections for colon cancer that were performed laparoscopically increased from 2.4% to 27.5%; the corresponding increase for elective resections for rectal cancer that were performed laparoscopically was from 1.1% to 21.5% (Box 3).
There was an increase in the percentage of laparoscopic resections across all hospital types and for cancer of both the colon and rectum, with the largest increases in high-volume private hospitals (Box 4). For both colon and rectal cancer, the rate of uptake of laparoscopic resection appeared to increase in 2003–04 (Box 5).
Laparoscopic colorectal resection is a very complex procedure, requiring mobilisation of a bulky structure, access to more than one quadrant of the abdomen, control of multiple large blood vessels, extraction of a large specimen, and successful creation of an anastomosis.9 Resection for malignant tumours has even more demanding requirements than resection for benign disease, because the surgeon must adhere to oncological principles — attainment of adequate surgical margins, removal of lymph nodes, proximal ligation of the vascular pedicles, minimal handling, and avoidance of perforation.9 Surgeons therefore need adequate training and experience to undertake laparoscopic resection for CRC.10
In Australia by 2007–08, about a quarter of elective resections for CRC were laparoscopic. Barring capacity constraints, it is likely that the percentage of laparoscopic resections will continue to increase because such minimally invasive surgery is probably feasible in about 90% of elective resections for CRC performed by experienced surgeons.11 Also, the number of elective resections are likely to increase as early detection of CRC through screening (either with faecal occult blood tests or colonoscopy) and the use of colonic stenting for obstruction reduce the need for emergency resections.
Compared with open resection, there is a much longer learning curve associated with laparoscopic resection for CRC; the required number of cases has been estimated to be up to 60–100.10,12 In Australia, uptake of laparoscopic resection for CRC across all hospital types is a reflection of continued postfellowship education and training of surgeons in this technique.
The training of surgeons and the assessment of their competency in laparoscopic CRC needs to be hierarchical because laparoscopic surgery for CRC involves a range of complexities, which are dependent on the anatomical site. Training typically begins with segmental resections of the right or left colon and then progresses to the more difficult resections of the rectosigmoid colon, transverse colon, and extraperitoneal rectum. A surgeon’s application for credentialling at each level of laparoscopic colorectal surgery should be accompanied by evidence of appropriate experience in the relevant procedures in open surgery. It is important that emerging specialists are also proficient in performing open resections for emergency cases and in cases where the laparoscopic technique is relatively contraindicated, such as when there are bowel adhesions. Progression through the levels needs to rely on objective evaluations from teachers and peers.13
The increasing uptake of laparoscopic resection for CRC in Australia is probably related to both better equipment and increasing evidence that long-term oncological outcomes are equivalent to those of open resection. The ultrasonic tissue dissector was introduced around the late 1990s, and better endoscopic stapling devices and high-definition videoendoscopy were introduced in the early 2000s. These devices improved laparoscopic access, particularly for resections within the pelvis. The increase in laparoscopic surgery for CRC in low-volume hospitals indicates that smaller centres outside the major cities are acquiring the technical facilities to perform laparoscopic resections. For both colon and rectal cancer, the rate of uptake increased in 2003–04. Publication of the Clinical Outcomes of Surgical Therapy trial14 for colon cancer and of the first randomised data for rectal cancer15 might have contributed to this.
We could only find one other study of population-based trends in the rates of laparoscopic resection for cancers of the colon and rectum. That publication, from the National Institute for Clinical Excellence in the United Kingdom, reported a percentage of laparoscopic procedures for elective resections for CRC of 9.0% in 2006–07,16 compared with 21.5% from our Australian study for the same year.
Four publications from the United States reported the percentage of laparoscopic resections for cancers of the colon only.17-20 These population-based studies used data from three different sources and reported a wide range in the percentage of CRC resections performed laparoscopically. Three of these studies reported a percentage of around 5%, which is very similar to that for Australia at the same time (2003–04).17,19,20 However, another study reported a percentage of 33.7% for the period 1 July 2004 to 30 June 2006,18 which is higher than the 14.3% for Australia over the same period (Box 3).
The short-term benefits of laparoscopic resection shown by international studies probably provide impetus for the uptake of this technique in Australia. Short-term outcomes from the Australian Laparoscopic Colon Cancer Surgical trial indicate that laparoscopic resection is associated with faster return of bowel function and shorter hospital stay.21 However, whether these and other short-term benefits such as reduced blood loss and better postoperative pulmonary function are being experienced outside the clinical trial environment would be of interest, and these questions should be the subject of future research. Also of interest in the real world of clinical practice is operating time. In a meta-analysis of randomised and non-randomised data, the mean operating time for laparoscopic surgery was 27% longer than for open surgery (175 minutes versus 147 minutes).3 Operating time is related to conversion rates, and it is possible that, as surgeons become more experienced with laparoscopic techniques, conversion rates will decrease and operating time will reduce to that of open surgery.
Impetus for the increased uptake of laparoscopic surgery for CRC comes not just from good quality evidence from randomised trials and recommendations by medical bodies,22-24 but also from the positive experiences of surgeons and their patients in everyday clinical practice. Given equivalent long-term oncological outcomes, patients with CRC prefer laparoscopic resections because of the proven short-term benefits.2
CRC is the most common internal cancer diagnosed in Australia.25 It is therefore likely that laparoscopic resection for CRC will be a procedure in high demand. The results from our article provide information to help the appropriate medical bodies achieve optimal success in the uptake of laparoscopic resection for CRC in Australia by providing the data necessary to ensure adequate resource allocation.
1 International statistical classification of diseases and related health problems. 10th revision, Australian modification (ICD-10-AM)8 codes used to define laparoscopic resections for colon and rectal cancer*
Additional ICD-10-AM codes indicating laparoscopic resection |
|||||||||||||||
* All cases included a principal diagnosis code of C18.0–C20.0. |
|||||||||||||||
2 Absolute numbers and percentage of elective resections for colorectal cancer by hospital volume and sector, Australia, 2000–01 and 2007–08
3 Laparoscopic resections as a percentage of all elective resections for colorectal cancer, Australia, 2000–01 and 2007–08
Provenance: Not commissioned; externally peer reviewed.
Received 16 September 2010, accepted 19 December 2010
- Bridie S Thompson1
- Michael D Coory3
- John W Lumley4
- 1 School of Population Health, University of Queensland, Brisbane, QLD.
- 2 Patient Safety and Quality Improvement Service, Queensland Health, Brisbane, QLD.
- 3 Cancer Council Victoria, Melbourne, VIC.
- 4 The Wesley Hospital, Brisbane, QLD.
We acknowledge the assistance of Corrie Martin, Principal Data Quality Officer of Queensland Health, and Julie Turtle, Clinical Coding Auditor/Educator of the Tasmanian Department Health and Human Services, for assisting with determining the International Statistical Classification of Diseases procedure codes relevant to segmental resections of the colon and rectum, and for clarifying coding practice for laparoscopic resections.
None identified.
- 1. Soper NJ, Brunt LM, Kerbl K. Laparoscopic general surgery. N Engl J Med 1994 10; 330: 409-419.
- 2. Schwenk W, Haase O, Neudecker J, et al. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev 2005; (3): CD003145.
- 3. Noel JK, Fahrbach K, Estok R, et al. Minimally invasive colorectal resection outcomes: short-term comparison with open procedures. J Am Coll Surg 2007; 204: 291-307.
- 4. Jackson TD, Kaplan GG, Arena G, et al. Laparoscopic versus open resection for colorectal cancer: a meta analysis of oncologic outcomes. J Am Coll Surg 2007; 204: 439-446.
- 5. Kuhry E, Schwenk W, Gaupset R, et al. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev 2008; 34: 498-504.
- 6. Breukink S, Pierie J, Wiggers T. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev 2007; (4): CD005200.
- 7. Australian Institute of Health and Welfare. National hospital morbidity database (NHMD). http://www.aihw.gov.au/national-hospital-morbidity-database/ (accessed Mar 2011).
- 8. National Centre for Classification in Health. International statistical classification of diseases and related health problems. 10th revision, Australian modification (ICD-10-AM). Sydney: NCCH, 2002.
- 9. Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Guidelines for laparoscopic resection of curable colon and rectal cancer. Los Angeles, Calif: SAGES, 2006. http://www.sages.org/publication/id/32/ (accessed Mar 2011).
- 10. Tekkis PP, Senagore AJ, Delaney CP, et al. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 2005; 242: 83-91.
- 11. Buchanan G, Malik A, Parvaiz A, et al. Laparoscopic resection for colorectal cancer. Br J Surg 2008; 95: 893-902.
- 12. Dinçler S, Koller MT, Steurer J, et al. Multidimensional analysis of learning curves in laparoscopic sigmoid resection: eight-year results. Dis Colon Rectum 2003; 46: 1371-1378; discussion 1378-1379.
- 13. Sachdeva AK, Russell TR. Safe introduction of new procedures and emerging technologies in surgery: education, credentialing, and privileging. Surg Clin North Am 2007; 87: 853-66, vi-vii.
- 14. Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004; 350: 2050-2059.
- 15. Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005; 365: 1718-1726.
- 16. National Institute for Clinical Excellence. NICE implementation uptake report: laparoscopic surgery for colorectal cancer. 2008. (NICE technology appraisal 105) http://www.nice.org.uk/media/411/18/ImplUptakeReportColorectalResectionsLaparoscopic.pdf (accessed Mar 2011).
- 17. Bilimoria KY, Bentrem DJ, Nelson H, et al. Use and outcomes of laparoscopic-assisted colectomy for cancer in the United States. Arch Surg 2008; 143: 832-839; discussion 839-840.
- 18. Delaney CP, Chang E, Senagore AJ, et al. Clinical outcomes and resource utilization associated with laparoscopic and open colectomy using a large national database. Ann Surg 2008; 247: 819-824.
- 19. Steele SR, Brown TA, Rush RM, et al. Laparoscopic vs open colectomy for colon cancer: results from a large nationwide population-based analysis. J Gastrointest Surg 2008; 12: 583-591.
- 20. Kemp JA, Finlayson SR. Nationwide trends in laparoscopic colectomy from 2000 to 2004. Surg Endosc 2008; 22: 1181-1187.
- 21. Hewett PJ, Allardyce RA, Bagshaw PF, et al. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg 2008; 248: 728-738.
- 22. Australian Cancer Network Colorectal Cancer Guidelines Revision Committee. Guidelines for the prevention, early detection and management of colorectal cancer. Sydney: Cancer Council Australia and Australian Cancer Network, 2005.
- 23. Veldkamp R, Gholghesaei M, Bonjer HJ, et al. Laparoscopic resection of colon cancer: consensus of the European Association of Endoscopic Surgery (EAES). Surg Endosc 2004; 18: 1163-1185.
- 24. National Institute for Health and Clinical Excellence. Laparoscopic surgery for colorectal cancer: review of NICE technology appraisal 17. London: NICE, 2006.
- 25. McDermid I. Cancer incidence projections Australia 2002 to 2011. Cat. no. CAN 25. Canberra: Australian Institute of Health and Welfare, Australasian Association of Cancer Registries and the National Cancer Strategies Group, 2005.





 3039000 or 3039300
3039000 or 3039300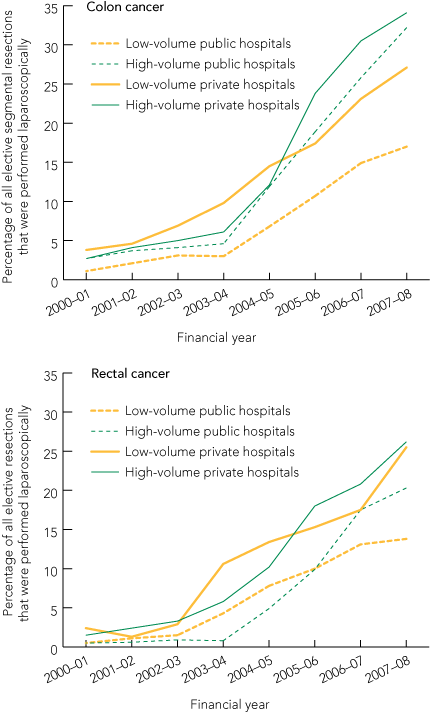
Abstract
Objective: To examine the trends in the uptake of laparoscopic resection for colorectal cancer.
Design and setting: Retrospective analysis of Australia-wide data on elective resections for colorectal cancer over the 8 financial years 2000–01 to 2007–08, obtained from the National Hospital Morbidity Database.
Main outcome measures: National trends in annual percentage of colorectal resections for cancer that were conducted laparoscopically for each year, stratified by hospitals conducting a high volume of elective resections (40 or more/year) versus a low volume, and by public versus private hospitals.
Results: For all Australian hospitals combined, the percentage of resections for colon cancer conducted laparoscopically increased from 2.4% in 2000–01 to 27.5% in 2007–08. For rectal cancer, this increase was from 1.1% to 21.5%. The largest increases were seen in high-volume private hospitals (colon cancer, 2.7% to 34.1%; rectal cancer, 1.5% to 26.2%), but increases also occurred in high-volume public hospitals (colon cancer, 2.7% to 32.2%; rectal cancer, 0.5% to 20.3%), low-volume private (colon cancer, 3.8% to 27.1%; rectal cancer, 2.4% to 25.5%) and low-volume public (colon cancer, 1.1% to 17.0%; rectal cancer, 0.5% to 13.8%) hospitals.
Conclusions: The use of laparoscopic resection for colorectal cancer has increased throughout Australian hospitals. Our findings provide the data necessary to ensure adequate resource allocation by the appropriate medical bodies to achieve optimal success in the uptake of laparoscopic resection for colorectal cancer in Australia.