Clinical record
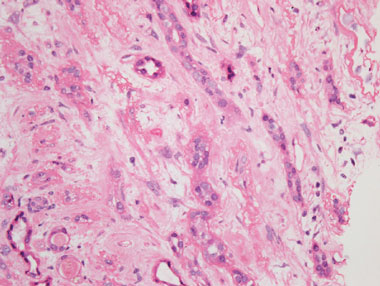
A renal ultrasound revealed unobstructed, small kidneys with cortical thinning bilaterally. A renal biopsy showed severe tubulointerstitial fibrosis and atrophy. There was no evidence of glomerulonephritis, nor of acute tubular necrosis (Box). These findings were consistent with chronic exposure to a nephrotoxin.
As the patient’s Chinese herbal products were suspected as the source of the nephrotoxin responsible for his nephropathy, they were sent to the Australian Therapeutic Goods Administration (TGA) to be analysed. High-performance liquid chromatography and mass spectrometry identified aristolochic acid in the Herbal International brand of long dan xie gan wan,1 and the product was subsequently recalled from the Australian market by the TGA.
In Australia, the use of complementary and alternative medicine (CAM), including Chinese herbal products, is increasing. However, scientific evidence on the safety, efficacy and quality of CAM, as well as regulatory controls, does not appear to support such popularity.2
Some herbal products contain aristolochic acid (AA), which is known to be nephrotoxic and carcinogenic.3 Aristolochic acid nephropathy (AAN), first reported in Belgium as “Chinese herbal nephropathy”,4 is characterised by progressive fibrosing interstitial nephritis leading to renal failure and severe anaemia. AAN is a worldwide problem, but its true incidence is unknown and probably underestimated,5 and though many cases of AAN have been reported, to our knowledge this is the first reported case in Australia. Many countries have prohibited the production and sale of herbal products containing AA,6 but despite bans, these products continue to be available through the internet or supplied through mail order.
In most of the reported cases, when the patient discontinued use of the drugs their renal disease still progressed rapidly to end stage.3 After exposure to AA, there is also a very high incidence of uroepithelial atypia and transitional cell carcinoma, and AA is now recognised as a potent urological carcinogen.7 Tissue samples from some patients with AAN and urothelial malignancy have revealed AA-related DNA adducts, which may be carcinogenic through a defect of DNA repair.8,9 The pathophysiological mechanisms of AAN are still unknown. In a rat AAN model, AA renal tubule toxicity has been associated with defective activation of antioxidative enzymes and mitochondrial damage. Activation of renal fibroblasts has also been proposed as the main source of collagen deposition leading to renal interstitial fibrosis.10
Therapeutic strategies have consisted mainly of supportive care in patients with AAN and renal failure. One study suggested that corticosteroids may slow the rate of renal deterioration.11 Renal transplantation may be an effective treatment strategy for those who progress to end-stage renal failure, with one report indicating no recurrence of AAN in five such patients after a follow-up period of 1 year.12
The clinical course of AAN has been related to the intensity and duration of AA exposure.13 A mean cumulative dose of 192 g of Aristolochia fangchi was ingested by one group of patients who developed end-stage renal failure related to AA.13 However, in most cases, the accumulated AA dose is difficult to ascertain because the dose is recalled retrospectively by patients; the patient may ingest herbal medicines irregularly and there is no strict recommendation of dosing on labels; the AA levels in the herbal mixtures vary due to different manufacturing processes; and the quantity of AA also varies in different herbs and even in the same herb grown in different areas.14 Also, patient factors such as genetic polymorphism, environmental chemicals and drugs may result in individual differences in susceptibility to AA toxicity.5
Many different brands of long dan xie gan wan are available and are commonly sold as a “liver tonic” containing the herb Caulis aristolochiae manshuriensis. This herb is known to contain AA, and there have been cases of nephropathy with similar clinical presentations reported after its ingestion.15 C. manshuriensis was not labelled as an ingredient in our patient’s brand of long dan xie gan wan. Although AA in long dan xie gan wan is the most likely aetiology for his nephropathy, other potential contributing nephrotoxins such as heavy metals and ochratoxin A, found in other products he was taking concomitantly, cannot be excluded.16,5
In Australia, the sale of CAM is regulated by the TGA.17 In July 2001, alerts to health practitioners were distributed by the TGA,18 and in January 2002, bans on herbal products suspected to contain AA were instituted,19 but this patient was still able to purchase the product by mail order. Chinese herbal medicines containing AA remain available for purchase over the internet and through Chinese herbal retailers.6,20 Internet commerce has revolutionised accessibility to herbal medicines and has made regulation more difficult. A South Australian public survey in 2004 found that about half of CAM users believed that CAM products were independently tested by the TGA, and did not report their use to their general practitioner.2 It is therefore apparent that tighter regulations on CAM are needed.
Renal tissue showing severe, diffuse interstitial fibrosis and tubular atrophy, with no evidence of significant interstitial inflammation
Lessons from practice
Herbal remedies can be sources of nephrotoxins.
Aristolochic acid found in herbal remedies can cause rapidly progressive interstitial nephritis, leading to end-stage kidney disease and urothelial malignancy, therefore requiring regular surveillance for abnormal urine cytology and cystoscopy.
In clinical assessments, clinicians need to enquire specifically about use of herbal products.
Drug regulatory authorities should maintain more stringent surveillance on herbal products.
Provenance: Not commissioned; externally peer reviewed.
- 1. Sorenson WR, Sullivan D. Determination of aristolochic acid I in botanicals and dietary supplements potentially contaminated with aristolochic acid I using LC-UV with confirmation by LC/MS: collaborative study. J AOAC Int 2007; 90: 925-933.
- 2. MacLennan AH, Myers SP, Taylor AW. The continuing use of complementary and alternative medicine in South Australia: costs and beliefs in 2004. Med J Aust 2006; 184: 27-31. <MJA full text>
- 3. Chang CH, Wang YM, Yang AH, Chiang SS. Rapidly progressive interstitial renal fibrosis associated with Chinese herbal medications. Am J Nephrol 2001; 21: 441-448.
- 4. Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including Chinese herbs. Lancet 1993; 341: 387-391.
- 5. Debelle FD, Vanherweghem JL, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int 2008; 74: 158-169.
- 6. Gold LS, Slone TH. Aristolochic acid, an herbal carcinogen, sold on the web after FDA alert. N Engl J Med 2003; 349: 1576-1577.
- 7. Nortier JL, Martinez MC, Schmeiser HH, et al. Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med 2000; 342: 1686-1692.
- 8. Schmeiser HH, Bieler CA, Wiessler M, et al. Detection of DNA adducts formed by aristolochic acid in renal tissue from patients with Chinese herbs nephropathy. Cancer Res 1996; 56: 2025-2028.
- 9. Cosyns JP, Jadoul M, Squifflet JP, et al. Urothelial lesions in Chinese-herb nephropathy. Am J Kidney Dis 1999; 33: 1011-1017.
- 10. Pozdzik AA, Salmon IJ, Debelle FD, et al. Aristolochic acid induces proximal tubule apoptosis and epithelial to mesenchymal transformation. Kidney Int 2008; 73: 595-607.
- 11. Martinez MC, Nortier J, Vereerstraeten P, Vanherweghem JL. Steroid therapy in chronic interstitial renal fibrosis: the case of Chinese-herb nephropathy. Nephrol Dial Transplant 2002; 17: 2033-2034.
- 12. Reginster F, Jadoul M, van Ypersele de Strihou C. Chinese herbs nephropathy presentation, natural history and fate after transplantation. Nephrol Dial Transplant 1997; 12: 81-86.
- 13. Martinez MC, Nortier J, Vereerstraeten P, Vanherweghem JL. Progression rate of Chinese herb nephropathy: impact of Aristolochia fangchi ingested dose. Nephrol Dial Transplant 2002; 17: 408-412.
- 14. Newall CA, Anderson LA, Phillipson JD. Herbal medicines: a guide for health care professionals. London: The Pharmaceutical Press, 1996.
- 15. Laing C, Hamour S, Sheaff M, et al. Chinese herbal uropathy and nephropathy. Lancet 2006; 368: 338.
- 16. Colson CR, De Broe ME. Kidney injury from alternative medicines. Adv Chronic Kidney Dis 2005; 12: 261-275.
- 17. Therapeutic Goods Administration. The regulation of complementary medicines in Australia — an overview. Canberra: Australian Government Department of Health and Ageing, 2006. http://www.tga.gov.au/cm/cmreg-aust.htm (accessed Feb 2011).
- 18. Therapeutic Goods Administration. Aristolochia alert for practitioners. Canberra: Australian Government Department of Health and Ageing, 2001. http://www.tga.gov.au/docs/html/arialert.htm (accessed Feb 2011).
- 19. Therapeutic Goods Administration. Long dan xie gan wan pill medicine recall. Canberra: Australian Government Department of Health and Ageing, 2002. http://www.tga.gov.au/recalls/2002/longxie.htm (accessed Feb 2011).
- 20. Cheung TP, Xue C, Leung K, et al. Aristolochic acids detected in some raw Chinese medicinal herbs and manufactured herbal products — a consequence of inappropriate nomenclature and imprecise labelling? Clin Toxicol (Phila) 2006; 44: 371-378.





None identified.